
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Apo-nefazodone
2. Dutonin
3. Lin-nefazodone
4. Menfazona
5. Nefadar
6. Nefazodone Hydrochloride
7. Rulivan
8. Serzone
1. 83366-66-9
2. Nefazodonum [latin]
3. Nefazodona [spanish]
4. Nefazodona
5. Nefazodonum
6. Nefazodone (inn)
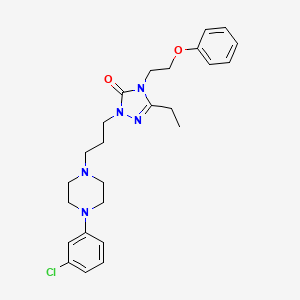
7. 2-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-5-ethyl-4-(2-phenoxyethyl)-1,2,4-triazol-3-one
8. 1-(3-(4-(m-chlorophenyl)-1-piperazinyl)propyl)-3-ethyl-4-(2-phenoxyethyl)-delta2-1,2,4-triazolin-5-one
9. Chembl623
10. 59h4fcv1tf
11. Chebi:7494
12. Nefadar
13. 1-(3-(4-(3-chlorophenyl)piperazin-1-yl)propyl)-3-ethyl-4-(2-phenoxyethyl)-1h-1,2,4-triazol-5(4h)-one
14. 2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}-5-ethyl-4-(2-phenoxyethyl)-2,4-dihydro-3h-1,2,4-triazol-3-one
15. Nefazodone [inn]
16. Nefazodone [inn:ban]
17. 1-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}-3-ethyl-4-(2-phenoxyethyl)-4,5-dihydro-1h-1,2,4-triazol-5-one
18. Smr000550487
19. Ncgc00165846-02
20. Unii-59h4fcv1tf
21. Sr-01000759312
22. 1-(3-(4-(3-chlorpheyl-1-piperazinylpropyl)-3-ethyl-4,5-dihydro-4-(2-phenoxyethyl)-1,2,4-triazol-5-on
23. Nefazodone [mi]
24. Nefazodone [vandf]
25. Nefazodone [who-dd]
26. Schembl35089
27. Mls001165769
28. Mls001195657
29. Bidd:gt0789
30. Gtpl7247
31. Dtxsid2023357
32. Hsdb 8411
33. Vitamind2,1alpha-hydroxy-
34. Hms2090d17
35. Hms2231i17
36. Hms3264k04
37. Hms3372c02
38. Pharmakon1600-01502314
39. Zinc538065
40. Bcp30990
41. Bdbm50069447
42. Nsc760344
43. Akos015907198
44. Ccg-213026
45. Db01149
46. Dutonin Pound>> Nefadar Pound>> Serzone
47. Ncgc00165846-01
48. Ncgc00165846-03
49. 2-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-ethyl-2,4-dihydro-4-(2-phenoxyethyl)-3h-1,2,4-triazol-3-one
50. 2-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-ethyl-4-(2-phenoxyethyl)-2h-1,2,4-triazol-3(4h)-one
51. 2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}-5-ethyl-4-[2-(phenyloxy)ethyl]-2,4-dihydro-3h-1,2,4-triazol-3-one
52. 3h-1,2,4-triazol-3-one, 2,4-dihydro-2-3-(4-(3-chlorophenyl)-1-piperazinyl)propyl)-5-ethyl-4-(2-phenoxyethyl)-
53. 3h-1,2,4-triazol-3-one, 2-(3-(4-(3-chlorophenyl)-1-piperazinyl)propyl)-5-ethyl-2,4-dihydro-4-(2-phenoxyethyl)-
54. Hy-119209
55. Cs-0076994
56. Ft-0654783
57. C07256
58. D08257
59. Ab00640019-14
60. Ab00640019_15
61. 366n669
62. A840565
63. L001196
64. Q416632
65. Sr-01000759312-5
66. Brd-k90789829-003-03-3
67. 1-(3-(4-(m-chlorophenyl)-1-piperazinyl)propyl)-3-ethyl-4-(2-phenoxyethyl)-.delta.(sup 2)-1,2,4-triazolin-5-one
68. 2-(3-[4-(3-chlorophenyl)-1-piperazinyl]propyl)-5-ethyl-4-(2-phenoxyethyl)-2,4-dihydro-3h-1,2,4-triazol-3-one #
69. 2-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-ethyl-4-(2-phenoxyethyl)-1,2,4-triazol-3-one
70. 3h-1,2,4-triazol-3-one, 2-(3-(4-(3-chlorophenyl)-1-piperazinyl))propyl)-5-ethyl-2,4-dihydro-4-(2-phenoxyethyl)-
| Molecular Weight | 470.0 g/mol |
|---|---|
| Molecular Formula | C25H32ClN5O2 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 469.2244530 g/mol |
| Monoisotopic Mass | 469.2244530 g/mol |
| Topological Polar Surface Area | 51.6 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 649 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antidepressive Agents, Second-Generation
National Library of Medicine's Medical Subject Headings. Nefazodone. Online file (MeSH, 2018). Available from, as of March 7, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Nefazodone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 7, 2018: https://clinicaltrials.gov/
Nefazodone hydrochloride tablets are indicated for the treatment of depression. When deciding among the alternative treatments available for this condition, the prescriber should consider the risk of hepatic failure associated with nefazodone hydrochloride treatment. In many cases, this would lead to the conclusion that other drugs should be tried first. /Included in US product label/
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
/BOXED WARNING/ Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of nefazodone hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Nefazodone hydrochloride tablets are not approved for use in pediatric patients.
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
/BOXED WARNING/ Cases of life-threatening hepatic failure have been reported in patients treated with nefazodone hydrochloride tablets. The reported rate in the United States is about 1 case of liver failure resulting in death or transplant per 250,000 to 300,000 patient-years of nefazodone hydrochloride treatment. The total patient-years is a summation of each patient's duration of exposure expressed in years. For example, 1 patient-year is equal to 2 patients each treated for 6 months, 3 patients each treated for 4 months, etc. Ordinarily, treatment with nefazodone hydrochloride tablets should not be initiated in individuals with active liver disease or with elevated baseline serum transaminases. There is no evidence that pre-existing liver disease increases the likelihood of developing liver failure, however, baseline abnormalities can complicate patient monitoring. Patients should be advised to be alert for signs and symptoms of liver dysfunction (jaundice, anorexia, gastrointestinal complaints, malaise, etc.) and to report them to their doctor immediately if they occur. Nefazodone hydrochloride tablets should be discontinued if clinical signs or symptoms suggest liver failure. Patients who develop evidence of hepatocellular injury such as increased serum AST or serum ALT levels >/= 3 times the upper limit of NORMAL, while on nefazodone hydrochloride tablets should be withdrawn from the drug. These patients should be presumed to be at increased risk for liver injury if nefazodone hydrochloride is reintroduced. Accordingly, such patients should not be considered for re-treatment.
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
The most commonly observed adverse events associated with the use of nefazodone (incidence of 5% or greater) and not seen at an equivalent incidence among placebo-treated patients (i.e., significantly higher incidence for nefazodone compared to placebo, p
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
Approximately 16% of the 3496 patients who received nefazodone in worldwide premarketing clinical trials discontinued treatment due to an adverse experience. The more common (>/= 1%) events in clinical trials associated with discontinuation and considered to be drug related (i.e., those events associated with dropout at a rate approximately twice or greater for nefazodone compared to placebo) included: nausea (3.5%), dizziness (1.9%), insomnia (1.5%), asthenia (1.3%), and agitation (1.2%).
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
For more Drug Warnings (Complete) data for Nefazodone (17 total), please visit the HSDB record page.
For the treatment of depression.
FDA Label
Nefazodone, an antidepressant synthetically derived phenylpiperazine, is used to treat major depression. Although it is structurally similar to trazodone, nefazodone has a mechanism of action different from other antidepressants and, hence, lacks the risk for major cardiovascular toxicity seen with tricyclics and insomnia and inhibition of REM sleep seen with the selective serotonin reuptake inhibitors.
Serotonin 5-HT2 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT2 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT2 RECEPTOR AGONISTS. Included under this heading are antagonists for one or more specific 5-HT2 receptor subtypes. (See all compounds classified as Serotonin 5-HT2 Receptor Antagonists.)
Serotonin and Noradrenaline Reuptake Inhibitors
Drugs that selectively block or suppress the plasma membrane transport of SEROTONIN and NORADRENALINE into axon terminals and are used as ANTIDEPRESSIVE AGENTS. (See all compounds classified as Serotonin and Noradrenaline Reuptake Inhibitors.)
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX06 - Nefazodone
Absorption
Nefazodone is rapidly and completely absorbed. Its absolute bioavailability is low (about 20%).
Route of Elimination
Nefazodone is extensively metabolized after oral administration by n-dealkylation and aliphatic and aromatic hydroxylation, and less than 1% of administered nefazodone is excreted unchanged in urine.
Volume of Distribution
0.22 to 0.87 L/kg
/MILK/ In two ... subjects, one taking 50 mg twice daily and the other 50 mg in the morning and 100 mg in the evening, the trough plasma levels were <50 ng/mL, whereas the paired milk concentrations were 687 and 213 ng/mL, respectively.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Tenth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2015, p. 964
/MILK/ ... In one woman taking 200 mg twice daily /of nefazodone/, the paired concentrations of nefazodone in milk and plasma (trough) were 57 and 617 ng/mL, respectively. the milk:plasma ratio was 0.09.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Tenth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2015, p. 964
/MILK/ Nefazodone is excreted into breast milk.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Tenth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2015, p. 964
/MILK/ The purpose of this study was/ to investigate whether adverse effects in a premature neonate could be attributed to nefazodone exposure via breast milk. The breast-fed white infant (female, 2.1 kg, 36 weeks corrected gestational age) of a 35-year-old woman (60 kg) taking nefazodone 300 mg/day was admitted to the hospital because she was drowsy, lethargic, unable to maintain normal body temperature, and was feeding poorly. A diagnosis of exposure to nefazodone via breast milk was considered only after other more likely diagnoses had been excluded. ... The maternal plasma and milk concentration-time profiles for nefazodone and its metabolites, triazoledione, HO-nefazodone, and m-chlorphenylpiperazine, were quantified by HPLC. The calculated infant dose for nefazodone and its active metabolites (as nefazodone equivalents) via the milk was only 0.45% of the weight-adjusted maternal nefazodone daily dose. ...
PMID:11098340 Yapp P et al; Ann Pharmacother 34 (11): 1269-72 (2000)
For more Absorption, Distribution and Excretion (Complete) data for Nefazodone (7 total), please visit the HSDB record page.
Hepatic.
... A 16-year-old female took 2.4 g of nefazodone. ... The terminal elimination half-life for nefazodone was 8.3 hours, and its metabolite hydroxy(OH)-nefazodone was 14.6 hours. BP-time curves demonstrated an 18-hour period of hypotension. There was a significant correlation between systolic BP and OH-nefazodone (R2 = 0.602). HR remained between 56 and 66 bpm for 30 hours despite hypotension. QT was significantly correlated with nefazodone (R2 = 0.911) and OH-nefazodone (R2 = 0.797), but no significant relationship between QTc and drug concentrations. ...
PMID:12733855 Isbister GK, Hackett LP; J Toxicol Clin Toxicol 41 (2): 167-73 (2003)
The purpose of this study was/ to investigate whether adverse effects in a premature neonate could be attributed to nefazodone exposure via breast milk. The breast-fed white infant (female, 2.1 kg, 36 weeks corrected gestational age) of a 35-year-old woman (60 kg) taking nefazodone 300 mg/day was admitted to the hospital because she was drowsy, lethargic, unable to maintain normal body temperature, and was feeding poorly. ... The maternal plasma and milk concentration-time profiles for nefazodone and its metabolites, triazoledione, HO-nefazodone, and m-chlorphenylpiperazine, were quantified by HPLC. The calculated infant dose for nefazodone and its active metabolites (as nefazodone equivalents) via the milk was only 0.45% of the weight-adjusted maternal nefazodone daily dose. ...
PMID:11098340 Yapp P et al; Ann Pharmacother 34 (11): 1269-72 (2000)
The utility of multivariate analysis in in vitro metabolite identification studies was examined with nefazodone, an antidepressant drug with a well-established metabolic profile. The chromatographic conditions were purposefully chosen to reflect those utilized in high-throughput screening for microsomal stability of new chemical entities. Molecular ion, retention time information on groups of human liver microsomal samples with/without nefazodone was evaluated by principal component analysis (PCA). Resultant scores and loadings plots from the PCA revealed the segregation and the ions of interest that designated the drug and its corresponding metabolites. Subsequent acquisition of tandem mass spectrometry (MS/MS) spectra for targeted ions permitted the interrogation and interpretation of spectra to identify nefazodone and its metabolites. A comparison of nefazodone metabolites identified by PCA versus those found by traditional metabolite identification approaches resulted in very good correlation when utilizing similar analytical methods. Fifteen metabolites of nefazodone were identified in beta-nicotinamide adenine dinucleotide phosphate (NADPH)-supplemented human liver microsomal incubations, representing nearly all primary metabolites previously reported. Of the 15 metabolites, eight were derived from the N-dealkylation and N-dephenylation of the N-substituted 3-chlorophenylpiperazine motif in nefazodone, six were derived from mono- and bis-hydroxylation, and one was derived from the Baeyer Villiger oxidation of the ethyltriazolone moiety in nefazodone.
PMID:20178453 Schneider RP et al; Xenobiotica 40 (4): 262-74 (2010)
Nefazodone is extensively metabolized after oral administration by n-dealkylation and aliphatic and aromatic hydroxylation, and less than 1% of administered nefazodone is excreted unchanged in urine. Attempts to characterize three metabolites identified in plasma, hydroxynefazodone (HO-NEF), meta-chlorophenylpiperazine (mCPP), and a triazole-dione metabolite, have been carried out.
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
This paper describes the complete profiling and characterization of in vitro metabolites of the antidepressant agent nefazodone (NEF) generated by human liver microsome (HLM). Two new metabolic pathways (biotransformation) for NEF have been discovered by the characterization of three new metabolites, including two new metabolites (M24, M25) formed due to the N-dealkylation reaction that occurred between the triazolone and propyl units, and one new metabolite (M26) formed due to the O-dearylation reaction that occurred on the phenoxyethyl unit. These metabolites were initially detected by a 4000 Q-Trap instrument and then confirmed by exact mass measurement using an LTQ-Orbitrap. Both instruments proved to be capable of providing complete in vitro metabolite information in a single liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis, although each had its advantages and disadvantages. One noticeable disadvantage of the 4000 Q-Trap was the reduced quality of isotopic pattern in the enhanced mass scan (EMS) spectrum when it was used as survey scan to trigger multiple dependent product ion scans. The problem was especially exacerbated for minor metabolites with low signal intensity. On the other hand, the LTQ-Orbitrap maintained excellent isotopic pattern when used as a full scan survey scan. Twenty-six metabolites were detected and identified. The formation of these new metabolites was also confirmed by analyzing duplicate incubations at different time points.
PMID:18000840 Li AC et al; Rapid Commun Mass Spectrom 21 (24): 4001-8 (2007)
Nefazodone has known human metabolites that include 1-(3-Chlorophenyl)piperazine, 5-Ethyl-4-(2-phenoxyethyl)-2-(3-hydroxypropyl)-2H-1,2,4-triazol-3(4H)-one, Hydroxynefazodone, and P-Hydroxynefazodone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
2-4 hours
... A 16-year-old female took 2.4 g of nefazodone. ... The terminal elimination half-life for nefazodone was 8.3 hours, and its metabolite hydroxy(OH)-nefazodone was 14.6 hours.
PMID:12733855 Isbister GK, Hackett LP; J Toxicol Clin Toxicol 41 (2): 167-73 (2003)
... In a 4-way crossover design, 16 subjects received clinically relevant doses of venlafaxine, nefazodone, or sertraline for 8 days or fluoxetine for 11 days. Treatments were separated by a 7- to 14-day washout period and fluoxetine was always the last antidepressant taken. ... Nefazodone was also the only antidepressant that caused a significant change in alprazolam (ALPZ) disposition, decreasing its area under the concentration-versus-time curve (AUC; P < 0.01), and increasing its elimination half-life (16.4 vs. 12.3 hours; P < 0.05) compared with values at baseline. ...
PMID:14709940 DeVane CL et al; J Clin Psychopharmacol 24 (1): 4-10 (2004)
... the half-life of nefazodone is 2 to 4 hours.
NIH; DailyMed. Current Medication Information for Nefazodone Hydrochloride Tablet (Updated: January 29, 2018). Available from, as of March 22, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=916f119c-836f-dbec-45e0-047c0f527925
Within the serotonergic system, nefazodone acts as an antagonist at type 2 serotonin (5-HT2) post-synaptic receptors and, like fluoxetine-type antidepressants, inhibits pre-synaptic serotonin (5-HT) reuptake. These mechanisms increase the amount of serotonin available to interact with 5-HT receptors. Within the noradrenergic system, nefazodone inhibits norepinephrine uptake minimally. Nefazodone also antagonizes alpha(1)-adrenergic receptors, producing sedation, muscle relaxation, and a variety of cardiovascular effects. Nefazodone's affinity for benzodiazepine, cholinergic, dopaminergic, histaminic, and beta or alpha(2)-adrenergic receptors is not significant.
Global Sales Information

ANALYTICAL

ABOUT THIS PAGE
75
PharmaCompass offers a list of Nefazodone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nefazodone manufacturer or Nefazodone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nefazodone manufacturer or Nefazodone supplier.
PharmaCompass also assists you with knowing the Nefazodone API Price utilized in the formulation of products. Nefazodone API Price is not always fixed or binding as the Nefazodone Price is obtained through a variety of data sources. The Nefazodone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nefazodone Hcl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nefazodone Hcl, including repackagers and relabelers. The FDA regulates Nefazodone Hcl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nefazodone Hcl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nefazodone Hcl manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nefazodone Hcl supplier is an individual or a company that provides Nefazodone Hcl active pharmaceutical ingredient (API) or Nefazodone Hcl finished formulations upon request. The Nefazodone Hcl suppliers may include Nefazodone Hcl API manufacturers, exporters, distributors and traders.
click here to find a list of Nefazodone Hcl suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nefazodone Hcl DMF (Drug Master File) is a document detailing the whole manufacturing process of Nefazodone Hcl active pharmaceutical ingredient (API) in detail. Different forms of Nefazodone Hcl DMFs exist exist since differing nations have different regulations, such as Nefazodone Hcl USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nefazodone Hcl DMF submitted to regulatory agencies in the US is known as a USDMF. Nefazodone Hcl USDMF includes data on Nefazodone Hcl's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nefazodone Hcl USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nefazodone Hcl suppliers with USDMF on PharmaCompass.
Nefazodone Hcl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nefazodone Hcl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nefazodone Hcl GMP manufacturer or Nefazodone Hcl GMP API supplier for your needs.
A Nefazodone Hcl CoA (Certificate of Analysis) is a formal document that attests to Nefazodone Hcl's compliance with Nefazodone Hcl specifications and serves as a tool for batch-level quality control.
Nefazodone Hcl CoA mostly includes findings from lab analyses of a specific batch. For each Nefazodone Hcl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nefazodone Hcl may be tested according to a variety of international standards, such as European Pharmacopoeia (Nefazodone Hcl EP), Nefazodone Hcl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nefazodone Hcl USP).

