



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
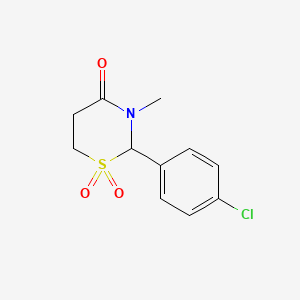

1. Chlormethazanone
1. 80-77-3
2. Trancopal
3. Chlormethazanone
4. Chlormethazone
5. Chlormezanon
6. Clormetazanone
7. Clormetazon
8. Phenarol
9. Dl-chlormezanone
10. Clorilax
11. Miorilax
12. Myolespen
13. Rilansyl
14. Rilaquil
15. Rilassol
16. Supotran
17. Trancote
18. Alinam
19. Banabin
20. Bisina
21. Tanafol
22. Rexan
23. Rilax
24. Banabin-sintyal
25. Muskel-trancopal
26. Mio-sed
27. Banabil-sintyal
28. 2-(4-chlorophenyl)-3-methyl-1,3-thiazinan-4-one 1,1-dioxide
29. Chlormezanonum
30. Dichloromezanone
31. Clormezanone [dcit]
32. Chlomedinon
33. Rillasol
34. Suprotan
35. Muskel
36. Lobak
37. (+-)-chlormezanone
38. Chlormezanonum [inn-latin]
39. Clormezanona [inn-spanish]
40. Dichloromethazanone
41. 2-(4-chlorophenyl)-3-methyl-1,1-dioxo-1,3-thiazinan-4-one
42. Win 4692
43. 2-(p-chlorophenyl)tetrahydro-3-methyl-4h-1,3-thiazin-4-one 1,1-dioxide
44. (+)-chlormezanone
45. (-)-chlormezanone
46. 4h-1,3-thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide
47. 2-(4-chlorophenyl)-3-methyl-4-metathiazanone-1,1-dioxide
48. (+)-fenarol
49. (-)-fenarol
50. 2-(p-chlorphenyl)-3-methyl-1,3-perhydrothiazin-4-on-1,1-dioxide
51. Chlormezanone, (+)-
52. Chlormezanone, (-)-
53. Chlormezanone (trancopal)
54. Tranrilax
55. Nsc-169108
56. 4bu37om8kl
57. 4h-1,3-thiazin-4-one, Tetrahydro-2-(p-chlorophenyl)-3-methyl-, 1,1-dioxide
58. C14wb33y0s
59. Chebi:3619
60. Clormezanone
61. Gp568v9g19
62. 2-(4-chlorophenyl)tetrahydro-3-methyl-4h-1,3-thiazin-4-one 1,1-dioxide
63. Chlormezanone 100 Microg/ml In Ethanol
64. Ncgc00015191-06
65. Ncgc00015191-07
66. Clormezanona
67. Rilasol
68. Transanate
69. (+-)-fenarol
70. Dsstox_cid_2798
71. Dsstox_rid_76732
72. Dsstox_gsid_22798
73. Chlormezanona
74. Chlormezanone [ban:inn:jan]
75. Chlormezanone [inn:ban:jan]
76. Mfcd00143951
77. 2-(4-chlorophenyl)tetrahydro-3-methyl-4h-1,3-thiazin-4-one-1,1-dioxide
78. 4h-1,3-thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide, (+)-
79. 4h-1,3-thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide, (-)-
80. 102818-66-6
81. 102818-67-7
82. Smr001456219
83. Hsdb 3300
84. Sr-01000075208
85. Trancopal (tn)
86. Einecs 201-307-4
87. Chlormezanone (jan/inn)
88. Nsc 169108
89. Fenaprim
90. Trancopa
91. 2-(4-chlorphenyl)-3-methyl-4-metathiazanon-1,1-dioxid
92. Unii-gp568v9g19
93. Cas-80-77-3
94. 4h-1,3-thiazin-4-one, 2-(p-chlorophenyl)tetrahydro-3-methyl-1,1-dioxide
95. Prestwick_736
96. 2-(para-chlorophenyl)tetrahydro-3-methyl-4h-1,3-thiazin-4-one, 1,1-dioxide
97. 4h-1,3-thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-1,1-dioxide
98. (+/-)-chlormezanone
99. Spectrum_000414
100. (.+/-.)-fenarol
101. Prestwick0_000336
102. Prestwick1_000336
103. Prestwick2_000336
104. Prestwick3_000336
105. Spectrum2_001807
106. Spectrum3_001084
107. Spectrum4_001237
108. Spectrum5_001364
109. C-192
110. Chlormezanone [mi]
111. (.+/-.)-chlormezanone
112. Biomol-nt_000277
113. Unii-4bu37om8kl
114. Chlormezanone [inn]
115. Chlormezanone [jan]
116. Chlormezanone [hsdb]
117. Unii-c14wb33y0s
118. Lopac0_000383
119. Oprea1_275911
120. Bspbio_000371
121. Bspbio_002728
122. Chlormezanone [vandf]
123. Kbiogr_001734
124. Kbioss_000894
125. Chlormezanone Trancopal
126. Mls003876813
127. Mls004773972
128. Chlormezanone [mart.]
129. Divk1c_000886
130. Schembl217864
131. Spectrum2300062
132. Spbio_001793
133. Spbio_002292
134. Chlormezanone [who-dd]
135. Bpbio1_000409
136. Bpbio1_001189
137. Gtpl7323
138. Chembl1200714
139. Dtxsid3022798
140. Hms502m08
141. Kbio1_000886
142. Kbio2_000894
143. Kbio2_003462
144. Kbio2_006030
145. Kbio3_001948
146. Weqayvwkmwhejo-uhfffaoysa-
147. Ninds_000886
148. Hms1569c13
149. Hms2096c13
150. Hms3261m07
151. Hms3266a22
152. Hms3411m15
153. Hms3655n09
154. Hms3675m15
155. Hms3713c13
156. Hms3884e20
157. Pharmakon1600-02300062
158. Chlormezanone [orange Book]
159. Bcp14385
160. Hy-b0353
161. Wln: T6vn Dswtj B1 Cr Dg
162. Tox21_110093
163. Tox21_113151
164. Tox21_302197
165. Tox21_500383
166. Ccg-39616
167. Nsc169108
168. Nsc759569
169. S2021
170. Chlormezanone 1.0 Mg/ml In Methanol
171. 4h-1,3-thiazin-4-one, 2-(p-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide
172. Akos000121464
173. Akos016844064
174. Tox21_110093_1
175. Db01178
176. Lp00383
177. Nsc-759569
178. Sdccgsbi-0050370.p004
179. Idi1_000886
180. Ncgc00015191-03
181. Ncgc00015191-04
182. Ncgc00015191-05
183. Ncgc00015191-08
184. Ncgc00015191-09
185. Ncgc00015191-10
186. Ncgc00015191-13
187. Ncgc00015191-22
188. Ncgc00024597-02
189. Ncgc00024597-03
190. Ncgc00024597-04
191. Ncgc00024597-05
192. Ncgc00024597-06
193. Ncgc00255305-01
194. Ncgc00261068-01
195. 2-(p-chlorphenyl)-3-methyl-1,1-dioxide
196. Sbi-0050370.p003
197. Ab00052410
198. Eu-0100383
199. Ft-0623609
200. Sw196880-3
201. C76585
202. D00268
203. Ab00052410_04
204. Ab00052410_05
205. Q5102983
206. Sr-01000075208-1
207. Sr-01000075208-3
208. Sr-01000075208-5
209. Sr-01000075208-6
210. Brd-a20348246-001-05-9
211. Brd-a20348246-001-08-3
212. 2-(p-chlorophenyl)tetrahydro-3-methyl-4h-1,1-dioxide
213. Z271004890
214. 4h-1, 2-(4-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide
215. 4h-1, 2-(p-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide
216. 2-(4-chlorophenyl)-3-methyl-1lambda6,3-thiazinane-1,1,4-trione
217. 2-(p-chlorphenyl)-3-methyl-1,3-perhydrothiazin-4-one, 1,1-dioxide
| Molecular Weight | 273.74 g/mol |
|---|---|
| Molecular Formula | C11H12ClNO3S |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 273.0226421 g/mol |
| Monoisotopic Mass | 273.0226421 g/mol |
| Topological Polar Surface Area | 62.8 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 395 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Anxiety Agents; Muscle Relaxants, Central
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...ADMIN IV HAVE ESTABLISHED VALUE IN TREATING ACUTE MUSCLE SPASMS ASSOC WITH TRAUMA & INFLAMMATION. ...ALSO BENEFICIAL IN PRODUCING MUSCLE RELAXATION FOR CERTAIN ORTHOPEDIC MANIPULATIONS. ...MAY TEMPORARILY ABATE SOME OF SYMPTOMS OF CEREBRAL PALSY... /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 241
MANY AGENTS WITH MUSCLE RELAXANT PROPERTIES PRODUCE NOTABLE SEDATION IN ORDINARY ORAL DOSES. SUCH AGENTS ENJOY PARTICULARLY WIDE USE IN TREATMENT OF MUSCLE TENSION & PAINS ASSOC WITH ANXIETY STATES & PSYCHOSOMATIC DISORDERS. /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 241
MUSCLE RELAXANTS CAUSE SKELETAL MUSCULAR RELAXATION, WITHOUT LOSS OF CONSCIOUSNESS, AS RESULT OF SELECTIVE ACTION UPON CNS. ... ALL TYPES OF EXPTL HYPERTONIA & HYPERREFLEXIA...PRODUCED BY SPINAL OR SUPRASPINAL LESIONS, ARE DIMINISHED... ALSO...PROTECTION AGAINST...CONVULSIVE AGENTS... /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 239
...AS EFFECTIVE AS CHLORDIAZEPOXIDE IN TREATING MILD ANXIETY. MUSCULOSKELETAL DISORDERS IN WHICH ANXIETY & TENSION INTENSIFY SYMPTOMS MAY RESPOND TO ITS SEDATIVE EFFECT...DOES NOT APPEAR TO HAVE ANY SPECIFIC EFFECT ON SPASTICITY OR RIGIDITY ASSOC WITH ORG NEUROLOGIC DISORDERS.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 418
SOMETIMES METABOLITE DISCOLORS URINE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 860
UNTOWARD EFFECTS ARE GENERALLY MILD & OCCUR RELATIVELY INFREQUENTLY BUT, IN 2 CONTROLLED STUDIES, REACTIONS WERE MORE COMMON...THAN WITH CHLORDIAZEPOXIDE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 419
...PRODUCE SOME SEDATION, @ LEAST @ HIGHEST DOSES EMPLOYED CLINICALLY. /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 241
Used in the management of anxiety and in the treatment of muscle spasm.
Chlormezanone is a non-benzodiazepine muscle relaxant. It was discontinued worldwide in 1996 by its manufacturer due to confirmed serious and rare cutaneous reactions (toxic epidermal necrolysis).
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
M - Musculo-skeletal system
M03 - Muscle relaxants
M03B - Muscle relaxants, centrally acting agents
M03BB - Oxazol, thiazine, and triazine derivatives
M03BB02 - Chlormezanone
RAPIDLY ABSORBED FROM GI TRACT...EFFECT WITHIN 15 TO 30 MIN...DURATION OF ACTION OF 4 TO 6 HR...FREE DRUG & /4-CHLOROHIPPURIC ACID/ EXCRETED IN URINE /HUMAN, ORAL/...PRESENT IN HIGH CONCN IN KIDNEY, LIVER, MUSCLE, HEART, & BODY FAT, & IN LESSER CONCN IN LUNG & PLASMA /RATS, ORAL/.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1969
AFTER ORAL ADMIN OF (14)C-CHLORMEZANONE, ABOUT 74% OF DOSE WAS EXCRETED INTO URINE OF RATS WITHIN 24 HR & 21% INTO URINE OF MICE WITHIN 2 HR. BILIARY EXCRETION OF RADIOACTIVITY WAS ABOUT 10% OF DOSE IN RATS.
HAKUSUI H ET AL; XENOBIOTICA 8 (4) 229-38 (1978)
CHLORMEZANONE...IS EXCRETED UNCHANGED IN HUMAN URINE & DOG BILE. FORMATION OF 4-CHLOROHIPPURIC ACID, MAJOR URINARY METABOLITE IN MAN, INVOLVES NON-ENZYMIC HYDROLYSIS, FOLLOWED BY OXIDATION & CONJUGATION OF 4-CHLOROBENZALDEHYDE PRODUCT OF HYDROLYSIS.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 181
(14)C-CHLORMEZANONE, METABOLITES IN URINE OF RATS & MICE WERE P-CHLOROBENZOIC ACID, P-CHLOROHIPPURIC ACID, N-METHYL-P-CHLOROBENZAMIDE, 2-[N-METHYL-N-(P-CHLOROBENZOYL)]CARBAMOYLETHYLSULFONIC ACID, 3-SULFOPROPIONIC ACID & GLUCURONIDE OF P-CHLOROBENZOIC ACID.
HAKUSUI H ET AL; XENOBIOTICA 8 (4) 229-38 (1978)
Chlormezanone binds to central benzodiazepine receptors which interact allosterically with GABA receptors. This potentiates the effects of the inhibitory neurotransmitter GABA, increasing the inhibition of the ascending reticular activating system and blocking the cortical and limbic arousal that occurs following stimulation of the reticular pathways.
NEURONAL CONDUCTION, NEUROMUSCULAR TRANSMISSION, & MUSCLE EXCITABILITY ARE NOT DEPRESSED EXCEPT AFTER NEARLY LETHAL DOSES. PROMINENT EFFECT...IS TO DEPRESS SPINAL POLYSYNAPTIC REFLEXES PREFERENTIALLY OVER MONOSYNAPTIC REFLEXES. /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 240
IN ABSENCE OF DEFINITIVE STUDIES IT APPEARS REASONABLE TO ASCRIBE BENEFICIAL EFFECTS...TO THEIR SEDATIVE PROPERTIES. /CENTRALLY ACTING MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 241


