



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
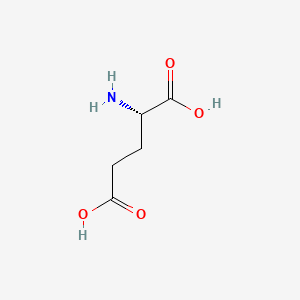

1. Aluminum L Glutamate
2. Aluminum L-glutamate
3. D Glutamate
4. D-glutamate
5. Glutamate
6. Glutamate, Potassium
7. Glutamic Acid, (d)-isomer
8. L Glutamate
9. L Glutamic Acid
10. L-glutamate
11. L-glutamate, Aluminum
12. L-glutamic Acid
13. Potassium Glutamate
1. L-glutamic Acid
2. 56-86-0
3. (2s)-2-aminopentanedioic Acid
4. (s)-2-aminopentanedioic Acid
5. Glutamidex
6. Glutaminol
7. H-glu-oh
8. L-glutamate
9. Glutacid
10. Glutaton
11. Aciglut
12. L-glutaminic Acid
13. Glutamicol
14. Glusate
15. (s)-glutamic Acid
16. L-glu
17. D-glutamiensuur
18. L-(+)-glutamic Acid
19. Acidum Glutamicum
20. Glutaminic Acid
21. Alpha-aminoglutaric Acid
22. Glutamic Acid, L-
23. Poly-l-glutamate
24. (s)-(+)-glutamic Acid
25. Acido Glutamico
26. Acide Glutamique
27. Fema No. 3285
28. Glut
29. Glutamate
30. L-alpha-aminoglutaric Acid
31. Alpha-glutamic Acid
32. Polyglutamic Acid
33. Glu
34. Pentanedioic Acid, 2-amino-, (s)-
35. 2-aminoglutaric Acid
36. Ccris 7314
37. Glutamic Acid (l-glutamic Acid)
38. 1-aminopropane-1,3-dicarboxylic Acid
39. L-2-aminoglutaric Acid
40. Acidum Glutaminicum
41. Ai3-18472
42. 25513-46-6
43. Epa Pesticide Chemical Code 374350
44. Nsc 143503
45. Glutamic Acid (van)
46. Glutamic Acid (h-3)
47. Glutaminic Acid (van)
48. Gamma-l-glutamic Acid
49. Glutamate, L-
50. Glutamic Acid [usan:inn]
51. Alpha-poly-l-glutamic Acid
52. L(+)-glutamic Acid
53. L-2-amino-pentanedioic Acid
54. Acide Glutamique [inn-french]
55. Acido Glutamico [inn-spanish]
56. Acidum Glutamicum [inn-latin]
57. Glutamic Acid, (s)-
58. Alpha-aminoglutaric Acid (van)
59. 3kx376gy7l
60. Ins No.620
61. E 620
62. Chebi:16015
63. Ins-620
64. Nsc-143503
65. 2-aminopentanedioic Acid, (s)-
66. Ncgc00024502-03
67. E620
68. Glutamic Acid Polymer
69. A-glutamic Acid
70. E-620
71. 55443-55-5
72. A-aminoglutaric Acid
73. Poly(alpha-l-glutamic Acid)
74. Alpha-l-glutamic Acid Polymer
75. L-a-aminoglutaric Acid
76. (2s)-2-aminopentanedioate
77. L-glutaminsaeure
78. L-acido Glutamico
79. .alpha.-glutamic Acid
80. Glt
81. L(+)-monosodium Glutamate Monohydrate
82. 1-amino-propane-1,3-dicarboxylic Acid
83. Glutamic Acid [usan]
84. 6106-04-3
85. Einecs 200-293-7
86. L-glutamic Acid (9ci)
87. Mfcd00002634
88. Aminoglutarate
89. Unii-3kx376gy7l
90. Alpha-glutamate
91. A-glutamate
92. L-gluatmate
93. A-aminoglutarate
94. L-glutamic-acid
95. L-glutamic Adid
96. 2-aminoglutarate
97. Aminoglutaric Acid
98. 1ftj
99. 1xff
100. (s)-glutamate
101. L-a-aminoglutarate
102. Alpha-aminoglutarate
103. Ggl
104. (l)-glutamic Acid
105. H-glu
106. L-glutamic,(s)
107. L-(+)-glutamate
108. Poly(l-glutamicacid)
109. L-alpha-aminoglutarate
110. Glutamic Acid (usp)
111. Poly(l-glutamic Acid)
112. Tocris-0218
113. [3h]-l-glutamic Acid
114. 1ii5
115. (+)-l-glutamic Acid
116. (s)-(+)-glutamate
117. (s)-glu
118. Dsstox_cid_659
119. L-[14c(u)]glutamate
120. (s)-2-aminopentanedioate
121. Biomol-nt_000170
122. Ec 200-293-7
123. Glutamic Acid [mi]
124. L-glutamic Acid (jp17)
125. Schembl2202
126. Dsstox_rid_75716
127. Gamma-poly(l-glutamic Acid)
128. Glutamic Acid [inn]
129. H-glu-2-chlorotrityl Resin
130. L-glutamic Acid-[13c5]
131. Dsstox_gsid_20659
132. L-glutamic Acid, 98.5%
133. Lopac0_000529
134. S)-2-aminopentanedioic Acid
135. Glutamic Acid [inci]
136. Glutamic Acid [vandf]
137. L-glutamic Acid [fcc]
138. L-glutamic Acid [jan]
139. Bpbio1_001132
140. Chembl575060
141. Gtpl1369
142. Hsdb 490
143. Glutamic Acid [usp-rs]
144. Glutamic Acid [who-dd]
145. L-glutamic Acid [fhfi]
146. L-glutamic Acid, 99%, Fcc
147. Dtxsid5020659
148. Bdbm17657
149. Chebi:53374
150. 1-aminopropane-1,3-dicarboxylate
151. Glutamic Acid, L- (7ci,8ci)
152. L (+)-glutamic Acid, Alpha-form
153. 1-amino-propane-1,3-dicarboxylate
154. 6899-05-4
155. L-glutamic Acid, Non-animal Source
156. Zinc1482113
157. Tox21_113053
158. Glutamic Acid [ep Monograph]
159. Hsci1_000269
160. Pdsp1_000128
161. Pdsp1_001539
162. Pdsp2_000127
163. Pdsp2_001523
164. S6266
165. Akos006238837
166. Akos015854087
167. Am81690
168. Ccg-204619
169. Db00142
170. Sdccgsbi-0050512.p002
171. Cas-56-86-0
172. Alanine Impurity B [ep Impurity]
173. Ncgc00024502-01
174. Ncgc00024502-02
175. Ncgc00024502-04
176. Ncgc00024502-07
177. (2s)-2-aminopentanedioic Acid;h-glu-oh
178. Ac-11294
179. Ds-13284
180. Gamma-poly(l-glutamic Acid) Macromolecule
181. Hy-14608
182. (s)-1-aminopropane-1,3-dicarboxylic Acid
183. (s)-2-amino-1,5-pentanedioic Acid
184. A6810
185. Cs-0003473
186. G0059
187. L-glutamic Acid, Bioultra, >=99.5% (nt)
188. L-glutamic Acid, Tested According To Ph.eur.
189. C00025
190. D00007
191. L-glutamic Acid, Nist(r)rm 8573, Usgs40
192. Lysine Acetate Impurity B [ep Impurity]
193. M02979
194. M03872
195. L-glutamic Acid, Jis Special Grade, >=99.0%
196. L-glutamic Acid, Nist(r) Rm 8574, Usgs41
197. 002g634
198. A831210
199. Sr-01000597730
200. J-502415
201. L-glutamic Acid, Reagentplus(r), >=99% (hplc)
202. L-glutamic Acid, Vetec(tm) Reagent Grade, >=99%
203. Sr-01000597730-1
204. L-glutamic Acid, >=99%, Fcc, Natural Sourced, Fg
205. Q26995161
206. F8889-8668
207. Z1250208666
208. 27322e29-9696-49c1-b541-86bef72de2f3
209. Glutamic Acid, European Pharmacopoeia (ep) Reference Standard
210. L-glutamic Acid, Certified Reference Material, Tracecert(r)
211. Glutamic Acid, United States Pharmacopeia (usp) Reference Standard
212. L-glutamic Acid, From Non-animal Source, Meets Ep Testing Specifications, Suitable For Cell Culture, 98.5-100.5%
213. L-glutamic Acid, Pharmagrade, Ajinomoto, Ep, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 147.13 g/mol |
|---|---|
| Molecular Formula | C5H9NO4 |
| XLogP3 | -3.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 147.05315777 g/mol |
| Monoisotopic Mass | 147.05315777 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 145 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Considered to be nature's "Brain food" by improving mental capacities; helps speed the healing of ulcers; gives a "lift" from fatigue; helps control alcoholism, schizophrenia and the craving for sugar.
In addition to being one of the building blocks in protein synthesis, it is the most widespread neurotransmitter in brain function, as an excitatory neurotransmitter and as a precursor for the synthesis of GABA in GABAergic neurons.
Absorption
Absorbed from the lumen of the small intestine into the enterocytes.Absorption is efficient and occurs by an active transport mechanism.
/MILK/ Previous short observational studies on the free amino acid (FAA) content of human milk have shown that glutamine and glutamic acid increase in the first 4 to 6 weeks of life. Changes in human milk content of free amino acids (FAAs) was determined at colostrum, 1 month, and 3 months of lactation in 16 healthy lactating women after delivery of full-term infants. Milk was collected at the end of each feeding (hindmilk) during 24 hours. Glutamic acid and taurine were the most abundant FAAs at colostrum. Although taurine remained stable throughout lactation, glutamic acid (the prevalent FAA) and glutamine increased approximately 2.5 and 20 times, respectively, with progressing lactation representing more than 50% of total FAA at 3 months. The content of essential FAA was also stable, so the change in total FAA content was almost entirely due to the changes in glutamic acid and glutamine. Breast-fed infants are supplied with progressively increasing amounts of glutamine and glutamic acid throughout lactation. The increasing intake of glutamic acid and glutamine could benefit breast-fed infants with molecules that are likely to protect the enteral mucosa and act as neurotransmitters and as a source of nitrogen.
PMID:11144435 Agostoni C et al; J Pediatr Gastroenterol Nutr. 31 (5): 508-12 (2000)
In this report, (13)N -labeled L-glutamine and L-glutamic acid was synthesized by an enzymatic method ... . Organ distribution studies and whole body scans in mongrel dogs demonstrated low myocardial uptake of glutamine and glutamic acid and that the liver demonstrated a greater uptake of glutamine than glutamic acid or ammonia.
Gelbard A et al; Radiology; 116:127-132 (1975)
The measurement of the intestinal metabolism of the nitrogen moiety of glutamic acid has been investigated by oral ingestion of l-[(15)N]glutamic acid and sampling of arterialized blood. Measurements have been made in six normal adults weighing an average of 72.8 kg ingesting 100 mg of l-[(15)N]glutamic acid after an overnight fast. Measurement of the enrichment of arterial glutamic acid, glutamine and alanine was by gas chromatography-mass spectrometry. Isotopic enrichment of the amino acids was followed for 150 min after the ingestion of the amino acid. Arterialized venous blood amino acid concentrations, measured by HPLC, demonstrated no significant changes during the course of the experiment. From the observed appearance of label in arterialized glutamic acid, alanine and glutamine, little luminal glutamic acid reaches the extracellular pool. The majority of the administered nitrogen label appears in the arterial alanine and glutamine components.
PMID:2908193 Johnson AW et al; Clin Sci (Lond). 75 (5): 499-502 (1988)
Hepatic
Cortical excitability reflects a balance between excitation and inhibition. Glutamate is the main excitatory and GABA the main inhibitory neurotransmitter in the mammalian cortex. Changes in glutamate and GABA metabolism may play important roles in the control of cortical excitability. Glutamate is the metabolic precursor of GABA, which can be recycled through the tricarboxylic acid cycle to synthesize glutamate. GABA synthesis is unique among neurotransmitters, having two separate isoforms of the rate-controlling enzyme, glutamic acid decarboxylase. The need for two separate genes on two chromosomes to control GABA synthesis is unexplained. Two metabolites of GABA are present in uniquely high concentrations in the human brain. Homocarnosine and pyrrolidinone have a major impact on GABA metabolism in the human brain. Both of these GABA metabolites have anticonvulsant properties and can have a major impact on cortical excitability. /Glutamate, GABA/
PMID:12467378 Petroff OA; Neuroscientist. 8 (6): 562-73 (2002)
The measurement of the intestinal metabolism of the nitrogen moiety of glutamic acid has been investigated by oral ingestion of l-[(15)N]glutamic acid and sampling of arterialized blood. Measurements have been made in six normal adults weighing an average of 72.8 kg ingesting 100 mg of l-[(15)N]glutamic acid after an overnight fast. Measurement of the enrichment of arterial glutamic acid, glutamine and alanine was by gas chromatography-mass spectrometry. Isotopic enrichment of the amino acids was followed for 150 min after the ingestion of the amino acid. Arterialized venous blood amino acid concentrations, measured by HPLC, demonstrated no significant changes during the course of the experiment. From the observed appearance of label in arterialized glutamic acid, alanine and glutamine, little luminal glutamic acid reaches the extracellular pool. The majority of the administered nitrogen label appears in the arterial alanine and glutamine components.
PMID:2908193 Johnson AW et al; Clin Sci (Lond). 75 (5): 499-502 (1988)
Glutamate activates both ionotropic and metabotropic glutamate receptors. The ionotropic ones being non-NMDA (AMPA and kainate) and NMDA receptors. Free glutamic acid cannot cross the blood-brain barrier in appreciable quantities; instead it is converted into L-glutamine, which the brain uses for fuel and protein synthesis. It is conjectured that glutamate is involved in cognitive functions like learning and memory in the brain, though excessive amounts may cause neuronal damage associated in diseases like amyotrophic lateral sclerosis, lathyrism, and Alzheimer's disease. Also, the drug phencyclidine (more commonly known as PCP) antagonizes glutamate at the NMDA receptor, causing behavior reminiscent of schizophrenia. Glutamate in action is extremely difficult to study due to its transient nature.


