
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
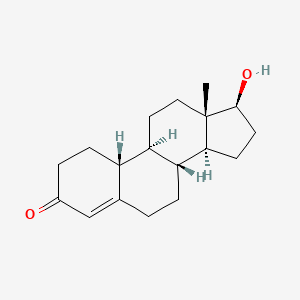

1. 17-hydroxy-estr-4-ene-3-one
2. 17beta Hydroxy 19 Nor 4 Androsten 3 One
3. 17beta-hydroxy-19-nor-4-androsten-3-one
4. 19-nortestosterone
5. Estrenolone
6. Norandrostenolone
7. Nortestosterone
1. 19-nortestosterone
2. 434-22-0
3. 19-norandrostenolone
4. Norandrostenolone
5. Nortestosterone
6. Menidrabol
7. 17beta-hydroxy-4-estren-3-one
8. 4-estren-17beta-ol-3-one
9. Nortestosteronum
10. Nandrolon
11. Nortestonate
12. Nandrolona
13. Nandrolonum
14. Oestrenolon
15. Norandrostenolon
16. 17beta-hydroxy-19-nor-4-androsten-3-one
17. 17beta-hydroxyestr-4-en-3-one
18. (17beta)-17-hydroxyestr-4-en-3-one
19. 17-beta-hydroestr-4-en-3-one
20. Nandrolone Ciii
21. (17-beta)-17-hydroxyestr-4-en-3-one
22. Nandrolone (inn)
23. Nandrolone [inn]
24. (8r,9s,10r,13s,14s,17s)-17-hydroxy-13-methyl-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-one
25. Chembl757
26. 6pg9vr430d
27. Chebi:7466
28. Estr-4-en-3-one, 17-hydroxy-, (17b)-
29. Nsc 3351
30. Nsc-3351
31. Ncgc00159416-02
32. U 2410
33. Dsstox_cid_3350
34. (+)-19-nortestosterone
35. Dsstox_rid_76987
36. Dsstox_gsid_23350
37. Nandrolonum [inn-latin]
38. Nandrolona [inn-spanish]
39. Decadura
40. Decadura (tn)
41. Cas-434-22-0
42. Smr000058610
43. Hsdb 3368
44. Nandrolone [inn:ban]
45. Einecs 207-101-0
46. Estr-4-en-3-one, 17-hydroxy-, (17beta)-
47. Estr-4-en-3-one, 17beta-hydroxy-
48. 4-estren-17.beta.-ol-3-one
49. Unii-6pg9vr430d
50. Nandrolone Base
51. Ncgc00164479-01
52. 19-nor-testosterone
53. Biobol
54. Estr-4-en-3-one, 17-beta-hydroxy-
55. 17.beta.-nandrolone
56. Nandrolone [mi]
57. Nandrolone Decanoic Acid
58. Estr-4-en-3-one, 17-hydroxy-, (17.beta.)-
59. Enta[a]phenanthren-3-one
60. Nandrolone [hsdb]
61. 17.beta.-nortestosterone
62. Nandrolone [vandf]
63. Ec 207-101-0
64. Nandrolone [mart.]
65. Estr-4-en-3-one, 17-hydroxy-, (17-beta)-
66. Nandrolone [who-dd]
67. Schembl20140
68. Mls001423989
69. Mls002222325
70. 17-hydroxyestr-4-en-3-one
71. Nandrolone(19-nortestosterone)
72. Gtpl6949
73. Dtxsid7023350
74. Deca-durabolin (decanoate Ester)
75. Nandrolone Ciii [usp-rs]
76. Durabolin (phenylpropionate Ester)
77. Hms2051i04
78. Hms2098e05
79. Hms2272p06
80. Hms3715e05
81. Zinc3814379
82. Tox21_113167
83. Tox21_113199
84. Tox21_201212
85. Bdbm50080092
86. Lmst02010044
87. Akos015894933
88. Nandrolone 1.0 Mg/ml In Acetonitrile
89. Tox21_113167_1
90. Ccg-100835
91. Cs-1416
92. Db13169
93. Gs-6819
94. Nc00085
95. 4-estren-3-one-17.beta.-ol
96. Nandrolone 1000 Microg/ml In Methanol
97. Ncgc00159416-03
98. Ncgc00159416-04
99. Ncgc00258764-01
100. (8r,9s,10r,13s,14s,17s)-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one
101. Ac-15207
102. Ac-30575
103. Cpd000058610
104. Hy-17432
105. 19-nortestosterone, >=99.0% (hplc)
106. 19-nortestosterone 100 Microg/ml In Methanol
107. C07254
108. D08250
109. Nandrolone, Vetranal(tm), Analytical Standard
110. 434n220
111. Q421709
112. Sr-01000781257
113. Sr-01000781257-3
114. W-106227
115. Nandrolone Decanoate Impurity D [ep Impurity]
116. Nandrolone, British Pharmacopoeia (bp) Reference Standard
117. Nandrolone, United States Pharmacopeia (usp) Reference Standard
118. (1s,2r,10r,11s,14s,15s)-14-hydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one
119. (8~{r},9~{s},10~{r},13~{s},14~{s},17~{s})-13-methyl-17-oxidanyl-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1~{h}-cyclop
120. (8~{r},9~{s},10~{r},13~{s},14~{s},17~{s})-13-methyl-17-oxidanyl-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1~{h}-cyclopenta[a]phenanthren-3-one
121. (8r,10r,13s,17s)-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one
122. 6vw
123. Nandrolone Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 274.4 g/mol |
|---|---|
| Molecular Formula | C18H26O2 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 274.193280068 g/mol |
| Monoisotopic Mass | 274.193280068 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anabolic Steroids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Nandrolone decanoate ... /is/ indicated in conditions such as chronic infections, extensive surgery, corticosteroid-induced myopathy, decubitus ulcers, burns, or severe trauma, which require reversal of catabolic processes or protein-sparing effects. /This agent is/ ... adjunct to, and not replacement for, conventional treatment of these disorders. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Nandrolone decanoate is indicated for the treatment of anemia associated with renal insufficiency (and as adjuvant therapy for aplastic and sickle cell anemias /NOT included in US product labeling/) Adequate iron intake is necessary for maximum therapeutic response. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Nandrolone phenpropionate is indicated in the treatment of refractory deficient red cell production anemias. These may include aplastic anemia, myelofibrosis, myelosclerosis, agnogenic myeloid metaplasia, and hypoplastic anemias caused by malignancy or myelotoxic drugs. Anabolic steroid therapy should not replace other supportive measures. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
For more Therapeutic Uses (Complete) data for NANDROLONE (10 total), please visit the HSDB record page.
Use of anabolic steroids by athletes is not recommended. Objective evidence is conflicting and inconclusive as to whether these medications significantly increase athletic performance by increasing muscle strength. Weight gains reported by athletes are due in part to fluid retention, which is a potentially hazardous side effect of anabolic steroid therapy. The risk of other unwanted effects, such as testicular atrophy and suppression of spermatogenesis in males; menstrual disturbances and virilization, such as deepening of voice, development of acne, and unnatural growth of body hair in females; peliosis hepatis or other hepatotoxicity; and hepatic cancer outweigh and possible benefit received from anabolic steroids and make their use in athletes inappropriate. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Anabolic steroids are not recommended for use during pregnancy, since studies in animals have shown that anabolic steroids cause masculinization of the fetus. Risk-benefit must be carefully considered. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
Contraindications: Hypersensitivity to anabolic steroids; male patients with prostate or breast carcinoma; carcinoma of the breast in females with hypercalcemia; nephrosis; the nephrotic phase of nephritis; pregnancy; to enhance physical appearance or athletic performance. /Anabolic steroids/
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 334
Anabolic agents may accelerate epiphyseal maturation more rapidly than linear growth in children, and the effect may continue for 6 months after the drug has been stopped. Therefore, monitor therapy by x-ray studies at 6 month intervals to avoid the risk of compromising adult height. Safety and efficacy in children with hereditary angioedema or metastatic breast cancer (rarely found) have not be established. /Anabolic steroids/
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 334
For more Drug Warnings (Complete) data for NANDROLONE (16 total), please visit the HSDB record page.
Anabolic Agents
These compounds stimulate anabolism and inhibit catabolism. They stimulate the development of muscle mass, strength, and power. (See all compounds classified as Anabolic Agents.)
Androgens
Compounds that interact with ANDROGEN RECEPTORS in target tissues to bring about the effects similar to those of TESTOSTERONE. Depending on the target tissues, androgenic effects can be on SEX DIFFERENTIATION; male reproductive organs, SPERMATOGENESIS; secondary male SEX CHARACTERISTICS; LIBIDO; development of muscle mass, strength, and power. (See all compounds classified as Androgens.)
A - Alimentary tract and metabolism
A14 - Anabolic agents for systemic use
A14A - Anabolic steroids
A14AB - Estren derivatives
A14AB01 - Nandrolone
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA11 - Nandrolone
Metabolic studies of (14)c-labe nortestosterone were carried out in mice and in calves. Radioactivity was quickly eliminated mainly in urine and feces. 10 wk after admin residual levels were low. Rapid absorption and elimination may be important in vet use.
RICO AG ET AL; ANN RECH VET 8(2) 135 (1977)
It is not known whether anabolic steroids are distributed into breast milk. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
Isomers of 3-hydroxyestran-17-one estrane-3,17-diol were identified by gas-liq chromatography-mass spectrometry in urine of a crossbred horse given 19-nortestosterone im.
HOUGHTON E; XENOBIOTICA 7(11) 683 (1977)
Metabolic studies of (14)c-labeled nortestosterone were carried out in mice by macroscopic autoradiog and in calves by liq scintillation determination of excretion and organ distribution. Radioactivity quickly eliminated in urine and feces. 10 wk after admin residual levels were low.
RICO AG ET AL; ANN RECH VET 8(2) 135 (1977)
Functionally overloading rat soleus muscle by synergist ablation induces a rapid increase in mass. Muscle remodeling during the first week of overload is critical for the overload-induced growth. Anabolic steroid modulation of this overload-induced remodeling response is not well understood. The purpose of this study was to determine whether pretreatment with nandrolone decanoate, a clinically administered anabolic steroid, alters muscle morphology and gene expression related to muscle growth during the initiation of functional overload in the rat soleus muscle. Adult (5 mo) male Fisher 344 x Brown Norway rats were randomly assigned to control (Sham), 3-day functional overload (OV), nandrolone decanoate administration (ND), or 3-day functional overload with nandrolone decanoate administration (OV+ND) treatment groups. Morphologically, OV increased the percentage of small (361%) and large (150%) fibers and expanded the ECM 50%. ND administration decreased the 3-day OV induction of small fibers 51% and nuclei associated with the ECM 20%. ND administration also attenuated the induction of cell cycle regulator p21 (64%) and myogenin (37%) mRNAs after 3 days of overload. These data demonstrate that nandrolone decanoate pretreatment can alter morphological and cell cycle regulator expression related to muscle growth at the onset of functional overload.
PMID:15886356 McClung JM et al; Am J Physiol Regul Integr Comp Physiol 288 (6): R1543-52 (2005)
Anabolic-androgenic steroids (AASs) are widely abused by adolescents, although persistent AAS use can cause several adverse physical and mental effects, including drug dependence. The first aim of the present study was to study the action of nandrolone decanoate on dopaminergic and serotonergic activities in the brains of rats. In order to evaluate the anabolic or toxic effects of the dosing regimens used, selected peripheral effects were monitored as well. Male Wistar rats were treated for 2 weeks. Injections containing nandrolone (5 and 20 mg/kg, i.m.) or vehicle were given once daily, 5 days a week. The levels of dopamine (DA), 5-hydroxytryptamine (5-HT) and their metabolites were assayed from dissected brain regions 3 days after the last injection. Blood was collected for chemical assays before, after 1 week treatment and at decapitation. Both doses of nandrolone significantly increased the levels of 3,4-dihydroxyphenylacetic acid (DOPAC), a metabolite of DA in the cerebral cortex, and the higher dose of nandrolone increased the concentrations of 5-HT in the cerebral cortex compared with the vehicle. In addition, after nandrolone treatment, the levels of hemoglobin and erythrocytes increased, and reticulocyte levels decreased. The results suggest that nandrolone at supraphysiological doses, high enough to induce erythropoiesis, induces changes in the dopaminergic and serotonergic neuronal system in the brains of rats. These phenomena may account to some of the observed central stimulatory properties that have been reported following AAS abuse.
PMID:15862791 Kurling S et al; Brain Res 1044 (1): 67-75 (2005)
Reverses catabolic processes and negative nitrogen balance by promoting protein anabolism and stimulating appetite if there is concurrently a proper intake of calories and proteins. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
Antianemic: Anemias due to bone marrow failure: Increases production and urinary excretion of erythropoietin. Anemias due to deficient red cell production : Stimulates erythropoietin production and may have a direct action on bone marrow. Anemias associated with renal disease: increases hemoglobin and red blood cell volume. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
 Cerata Pharmaceuticals LLP: WHO-GMP Certified Leading Manufacturer & Exporter of Steroid-Hormone & Peptide APIs From India.
Cerata Pharmaceuticals LLP: WHO-GMP Certified Leading Manufacturer & Exporter of Steroid-Hormone & Peptide APIs From India.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Date of Issue : 2022-08-31
Valid Till : 2025-07-02
Written Confirmation Number : WC-0162Amended
Address of the Firm :


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Global Sales Information

ABOUT THIS PAGE
56
PharmaCompass offers a list of Nandrolone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nandrolone manufacturer or Nandrolone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nandrolone manufacturer or Nandrolone supplier.
PharmaCompass also assists you with knowing the Nandrolone API Price utilized in the formulation of products. Nandrolone API Price is not always fixed or binding as the Nandrolone Price is obtained through a variety of data sources. The Nandrolone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nandrolone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nandrolone, including repackagers and relabelers. The FDA regulates Nandrolone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nandrolone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nandrolone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nandrolone supplier is an individual or a company that provides Nandrolone active pharmaceutical ingredient (API) or Nandrolone finished formulations upon request. The Nandrolone suppliers may include Nandrolone API manufacturers, exporters, distributors and traders.
click here to find a list of Nandrolone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nandrolone written confirmation (Nandrolone WC) is an official document issued by a regulatory agency to a Nandrolone manufacturer, verifying that the manufacturing facility of a Nandrolone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nandrolone APIs or Nandrolone finished pharmaceutical products to another nation, regulatory agencies frequently require a Nandrolone WC (written confirmation) as part of the regulatory process.
click here to find a list of Nandrolone suppliers with Written Confirmation (WC) on PharmaCompass.
Nandrolone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nandrolone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nandrolone GMP manufacturer or Nandrolone GMP API supplier for your needs.
A Nandrolone CoA (Certificate of Analysis) is a formal document that attests to Nandrolone's compliance with Nandrolone specifications and serves as a tool for batch-level quality control.
Nandrolone CoA mostly includes findings from lab analyses of a specific batch. For each Nandrolone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nandrolone may be tested according to a variety of international standards, such as European Pharmacopoeia (Nandrolone EP), Nandrolone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nandrolone USP).

