



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
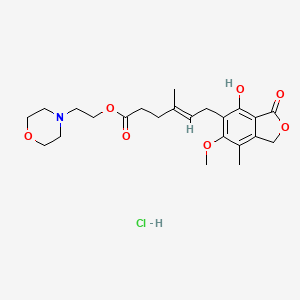

1. Cellcept
2. Mofetil Hydrochloride, Mycophenolate
3. Mofetil, Mycophenolate
4. Mycophenolate Mofetil
5. Mycophenolate Sodium
6. Mycophenolate, Sodium
7. Mycophenolic Acid
8. Mycophenolic Acid Morpholinoethyl Ester
9. Myfortic
10. Rs 61443
11. Rs-61443
12. Rs61443
13. Sodium Mycophenolate
1. 116680-01-4
2. Mycophenolate Mofetil Hcl
3. Mycophenolate Mofetil (hydrochloride)
4. Mycophenolate Mofetil Hydrochloride [usan]
5. Uxh81s8zvb
6. Rs-61443-190
7. Cellcept (tn)
8. 2-(4-morpholinyl)ethyl Ester (e)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic Acid, Hydrochloride
9. 2-morpholin-4-ylethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-2-benzofuran-5-yl)-4-methylhex-4-enoate;hydrochloride
10. 2-morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate Hydrochloride
11. 2-morpholin-4-ylethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-isobenzofuran-5-yl)-4-methyl-hex-4-enoate Hydrochloride
12. Mycophenolate Mofetil Hydrochloride (usan)
13. Unii-uxh81s8zvb
14. Rs 61443-190
15. Mycophenolatemofetilhydrochloride
16. Schembl1235420
17. Chembl1200955
18. Hy-b0199a
19. Dtxsid101027191
20. Akos000280984
21. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl Ester, Hydrochloride, (4e)-
22. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl Ester, Hydrochloride, (e)-
23. D05094
24. Mycophenolate Mofetil Hydrochloride [mart.]
25. Mycophenolate Mofetil Hydrochloride [vandf]
26. Mycophenolate Mofetil Hydrochloride [who-dd]
27. Q27291325
28. Mycophenolate Mofetil Hydrochloride [orange Book]
29. 2-morpholin-4-ylethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-isobenzofuran-5-yl)-4-methyl-hex-
| Molecular Weight | 470.0 g/mol |
|---|---|
| Molecular Formula | C23H32ClNO7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 469.1867301 g/mol |
| Monoisotopic Mass | 469.1867301 g/mol |
| Topological Polar Surface Area | 94.5 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 646 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Mycophenolate mofetil hydrochloride |
| Drug Label | CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6... |
| Active Ingredient | Mycophenolate mofetil hydrochloride |
| Dosage Form | Injectable |
| Route | injection |
| Strength | 500mg |
| Market Status | Tentative Approval |
| Company | Bedford Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Mycophenolate mofetil hydrochloride |
| Drug Label | CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6... |
| Active Ingredient | Mycophenolate mofetil hydrochloride |
| Dosage Form | Injectable |
| Route | injection |
| Strength | 500mg |
| Market Status | Tentative Approval |
| Company | Bedford Labs |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antibiotics, Antitubercular
Substances obtained from various species of microorganisms that are, alone or in combination with other agents, of use in treating various forms of tuberculosis; most of these agents are merely bacteriostatic, induce resistance in the organisms, and may be toxic. (See all compounds classified as Antibiotics, Antitubercular.)






