



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
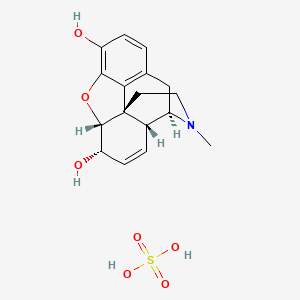

1. Chloride, Morphine
2. Contin, Ms
3. Duramorph
4. Morphia
5. Morphine
6. Morphine Chloride
7. Morphine Sulfate
8. Morphine Sulfate (2:1), Anhydrous
9. Morphine Sulfate (2:1), Pentahydrate
10. Ms Contin
11. Oramorph Sr
12. Sdz 202 250
13. Sdz 202-250
14. Sdz 202250
15. Sdz202 250
16. Sdz202-250
17. Sdz202250
18. Sulfate, Morphine
1. Morphine Sulfate
2. Schembl29317
3. Morphinesulfatenarcoticanalgesic
| Molecular Weight | 383.4 g/mol |
|---|---|
| Molecular Formula | C17H21NO7S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 383.10387318 g/mol |
| Monoisotopic Mass | 383.10387318 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 576 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Astramorph pf |
| PubMed Health | Morphine (Injection) |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 6 | |
|---|---|
| Drug Name | Duramorph pf |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Hikma Maple |
| 3 of 6 | |
|---|---|
| Drug Name | Morphine sulfate |
| PubMed Health | Morphine Sulfate Liposome (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | DESCRIPTIONChemically, morphine sulfate is 7, 8-didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol sulfate (2:1) (salt) pentahydrate... |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Capsule, extended release; Solution |
| Route | Intramuscular, intravenous; oral; Injection; Oral |
| Strength | 2mg/ml; 200mg; 10mg/5ml; 1mg/ml; 8mg/ml (8mg/ml); 30mg; 10mg/ml (10mg/ml); 100mg/5ml; 90mg; 15mg; 120mg; 4mg/ml (4mg/ml); 10mg/ml; 0.5mg/ml; 2mg/ml (2mg/ml); 75mg; 4mg/ml; 5mg/ml; 100mg; 5mg/ml (5mg/ml); 8mg/ml; 50mg; 60mg; 10mg; 15mg/ml; 20mg/5ml; 80mg; |
| Market Status | Prescription |
| Company | Clonmel Hlthcare; Vintage Pharms; Mylan Pharms; Upsher Smith; Hospira; Mallinckrodt; Rhodes Pharms; Meridian Medcl; Par Pharm; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Paddock; Caraco; Ranbaxy Labs; Bd Rx |
| 4 of 6 | |
|---|---|
| Drug Name | Astramorph pf |
| PubMed Health | Morphine (Injection) |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 5 of 6 | |
|---|---|
| Drug Name | Duramorph pf |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml; 0.5mg/ml |
| Market Status | Prescription |
| Company | Hikma Maple |
| 6 of 6 | |
|---|---|
| Drug Name | Morphine sulfate |
| PubMed Health | Morphine Sulfate Liposome (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | DESCRIPTIONChemically, morphine sulfate is 7, 8-didehydro-4, 5-epoxy-17-methylmorphinan-3, 6-diol sulfate (2:1) (salt) pentahydrate... |
| Active Ingredient | Morphine sulfate |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Capsule, extended release; Solution |
| Route | Intramuscular, intravenous; oral; Injection; Oral |
| Strength | 2mg/ml; 200mg; 10mg/5ml; 1mg/ml; 8mg/ml (8mg/ml); 30mg; 10mg/ml (10mg/ml); 100mg/5ml; 90mg; 15mg; 120mg; 4mg/ml (4mg/ml); 10mg/ml; 0.5mg/ml; 2mg/ml (2mg/ml); 75mg; 4mg/ml; 5mg/ml; 100mg; 5mg/ml (5mg/ml); 8mg/ml; 50mg; 60mg; 10mg; 15mg/ml; 20mg/5ml; 80mg; |
| Market Status | Prescription |
| Company | Clonmel Hlthcare; Vintage Pharms; Mylan Pharms; Upsher Smith; Hospira; Mallinckrodt; Rhodes Pharms; Meridian Medcl; Par Pharm; Roxane; Lannett Holdings; Nesher Pharms; Vistapharm; Actavis Elizabeth; Paddock; Caraco; Ranbaxy Labs; Bd Rx |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)








