



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
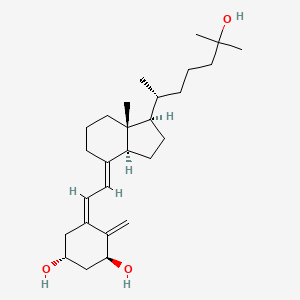

1. 1 Alpha, 25-dihydroxy-20-epi-vitamin D3
2. 1 Alpha,25 Dihydroxyvitamin D3
3. 1 Alpha,25-dihydroxycholecalciferol
4. 1 Alpha,25-dihydroxyvitamin D3
5. 1, 25-(oh)2d3
6. 1,25 Dihydroxy 20 Epi Vitamin D3
7. 1,25 Dihydroxycholecalciferol
8. 1,25 Dihydroxyvitamin D3
9. 1,25(oh)2-20epi-d3
10. 1,25(oh)2d3
11. 1,25-dihydroxy-20-epi-vitamin D3
12. 1,25-dihydroxycholecalciferol
13. 1,25-dihydroxyvitamin D3
14. 20-epi-1alpha,25-dihydroxycholecaliferol
15. Bocatriol
16. Calcijex
17. Calcitriol Kyramed
18. Calcitriol Nefro
19. Calcitriol-nefro
20. D3, 1 Alpha,25-dihydroxyvitamin
21. D3, 1,25-dihydroxy-20-epi-vitamin
22. D3, 1,25-dihydroxyvitamin
23. Decostriol
24. Kyramed, Calcitriol
25. Mc 1288
26. Mc-1288
27. Mc1288
28. Osteotriol
29. Renatriol
30. Rocaltrol
31. Silkis
32. Sitriol
33. Soltriol
34. Tirocal
1. 32222-06-3
2. Rocaltrol
3. Calcijex
4. Topitriol
5. 1alpha,25-dihydroxyvitamin D3
6. Silkis
7. 1alpha,25-dihydroxycholecalciferol
8. Soltriol
9. Vectical
10. 1,25-dhcc
11. 1,25-dihydroxycholecalciferol
12. 1,25-dihydroxyvitamin D3
13. 1,25-dihydroxyvitamin D
14. Calcitriolum
15. 1alpha,25(oh)2d3
16. Ro 21-5535
17. Dihydroxyvitamin D3
18. 1-alpha,25-dihydroxyvitamin D3
19. Dn-101
20. Chembl846
21. (1s,3r,5z,7e)-9,10-secocholesta-5,7,10-triene-1,3,25-triol
22. 1a,25-dihydroxyvitamin D3
23. Fxc9231jvh
24. Ro-21-5535
25. 1,25-hydroxylated Vitamin D
26. 1a,25-dihydroxycholecalciferol
27. Chebi:17823
28. 1-alpha-25-dihydroxyvitamin D3
29. Vit D
30. (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-1-[(2r)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol
31. (1r,3s,z)-5-((e)-2-((1r,3as,7ar)-1-((r)-6-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1h-inden-4(2h)-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol
32. Ro-215535
33. Ncgc00161327-04
34. Dn 101
35. (1alpha,3beta,5z,7e)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol
36. (5z,7e)-(1s,3r)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol
37. 9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (1a,3b,5z,7e)-
38. Ro 215535
39. 1,25-dihydroxy Vitamin D3
40. Toptriol
41. 1000873-74-4
42. 1alpha,25-dihydroxyvitamin D
43. Decostriol
44. (1s,3r,5z,7e)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol
45. (5z,7e)-(1s,3r)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol
46. Calcitriolum [inn-latin]
47. 1alpha,25-dihydroxyvitamin D3 / 1alpha,25-dihydroxycholecalciferol / Calcitriol
48. Calcitriol (rocaltrol)
49. 1,25-dihydroxycholecaliferol
50. Smr000466393
51. 1,25 (oh)2 D3
52. Ccris 5522
53. Dihydroxy-vitamin D3
54. Hsdb 3482
55. Einecs 250-963-8
56. Unii-fxc9231jvh
57. 1,25-(oh)2d3
58. Asentar
59. Panbonis
60. 1,25 Dihydroxycholecalciferol
61. 1,25-(oh)2-d3
62. 1.alpha.,25-dihydroxycholecalciferol
63. 9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta.,5z,7e)-
64. 1alpha 25-dihydroxycholecalciferol
65. Calcitriol Solution
66. Rocaltrol (tn)
67. (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-1-[(1r)-5-hydroxy-1,5-dimethyl-hexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylene-cyclohexane-1,3-diol
68. 5-{2-[1-(5-hydroxy-1,5-dimethyl-hexyl)-7a-methyl-octahydro-inden-4-ylidene]-ethylidene}-4-methylene-cyclohexane-1,3-diol
69. U 49562
70. Mfcd00867079
71. Calcitriol [usan:usp:inn:ban:jan]
72. Cholecalciferol, 1-alpha,25-dihydroxy-
73. 1db1
74. Starbld0021993
75. Calcitriol [mi]
76. Calcitriol [inn]
77. Calcitriol [jan]
78. Spectrum5_002061
79. Calcitriol [hsdb]
80. Calcitriol [usan]
81. 9,10-seco(5z,7e)-5,7,10(19)-cholestatriene-1alpha,3beta,25-triol
82. Dsstox_cid_2722
83. Calcitriol [vandf]
84. Synthetic Vitamin D Analog
85. 25-dihydroxycholecalciferol
86. 9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (1alpha,3beta,5z,7e)-
87. Calcitriol [mart.]
88. Schembl3245
89. Calcitriol [usp-rs]
90. Calcitriol [who-dd]
91. Dsstox_rid_76700
92. 1alpha,25(oh)2-d3
93. Dsstox_gsid_22722
94. Bspbio_001287
95. Calcitriol (jan/usp/inn)
96. Mls000759536
97. Mls001424122
98. 1
99. A,25-dihydroxyvitamin D3
100. Bml2-e03
101. Gtpl2779
102. (1s,3r,5z,7e)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol
103. Calcitriol [orange Book]
104. Dtxsid5022722
105. Bcbcmap01_000160
106. Chebi:93988
107. Calcitriol [ep Monograph]
108. Cas Number 32222-06-3
109. (5z,7e)-9,10-secocholesta-5,7,10(19)-triene-1.alpha.,3.beta.,25-triol
110. Bcpp000304
111. Calcitriol [usp Monograph]
112. Hms1361a09
113. Hms1791a09
114. Hms1989a09
115. Hms2051f06
116. Hms2089n03
117. Hms2232d18
118. Hms3402a09
119. 1 Alpha ,25-dihydroxyvitamin D3
120. Act06832
121. Ex-a4435
122. 1,25 Dihydroxy Vitamin D3
123. 1a,25-(oh)2d3
124. Tox21_111988
125. Bdbm50200182
126. Lmst03020258
127. Nsc749776
128. S1466
129. 1-alpha,-1,25-dihydroxyvitamin D3
130. (5z,7e)-9,10-secocholesta-5,7,10(19)-triene-1alpha,3beta,25-triol
131. Akos015961898
132. Zinc100015048
133. Ac-1859
134. Bcp9000474
135. Ccg-101001
136. Cd-2027
137. Cs-0388
138. Db00136
139. Nc00251
140. Nsc-749776
141. 1,25(oh2)d3
142. 1alpha,25-dihydroxyvitamin D3 Solution
143. Idi1_033757
144. 1,25(oh)2d3 & Cd4
145. Calcitrol 100 Microg/ml In Acetonitrile
146. Ncgc00161327-01
147. (1r,3s,z)-5-(2-((1r,3as,7ar,e)-1-((r)-6-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4h-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol
148. 1.alpha.,25-dihydroxyvitamin D(sub 3)
149. Cpd000466393
150. Hy-10002
151. Ro215535
152. Cas-32222-06-3
153. Vitamin D3-1alpha,25-dihydroxy (calcitriol)
154. 1,25d3
155. C01673
156. D00129
157. Ab00639957-06
158. Ab00639957_07
159. 1,25-dihydroxycholecalciferol, (1alpha)-
160. 1alpha,25-dihydroxyvitamin D3, >=99% (hplc)
161. 222c063
162. Q139195
163. Sr-01000759361
164. Sr-01000946978
165. 1alpha,25-dihydroxyvitamin D3, >=97.0% (hplc)
166. Sr-01000759361-4
167. Sr-01000946978-1
168. 1.alpha.,25-dihydroxy-26,27-hexadeuterovitamin D3
169. Brd-k27316855-001-06-7
170. Brd-k27316855-001-19-0
171. (3b,5z,7e)-9,10-secocholesta-5,7,10(19)-trienetriol
172. Calcitriol, European Pharmacopoeia (ep) Reference Standard
173. (1?,3?,5z,7e)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol
174. (5z,10-secocholesta-5,7,10(19)-triene-1.alpha.,3.beta.,25-triol
175. (1s,3r,5z,7e,14beta,17alpha)-9,10-secocholesta-5,7,10-triene-1,3,25-triol
176. 9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5z,7e)-
177. 9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5z,7e)- & Cd4
178. (1r,3s)-5-{2-[(1r,3as,7ar)-1-((r)-5-hydroxy-1,5-dimethyl-hexyl)-7a-methyl-octahydro-inden-4-ylidene]-ethylidene}-4-methylene-cyclohexane-1,3-diol
179. 1,3-cyclohexanediol, 4-methylene-5-[2-[(1r,3as,7ar)-octahydro-1-[(1r)-5-hydroxy-1,5-dimethylhexyl]-7a-methyl-4h-inden-4-ylidene]ethylidene]-, (1r,3s)-
| Molecular Weight | 416.6 g/mol |
|---|---|
| Molecular Formula | C27H44O3 |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 416.32904526 g/mol |
| Monoisotopic Mass | 416.32904526 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 688 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Calcijex |
| Drug Label | Calcijex (calcitriol injection) is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcijex is available in 1 mL ampuls. Each 1 mL contains calcitr... |
| Active Ingredient | Calcitriol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.001mg/ml; 0.002mg/ml |
| Market Status | Prescription |
| Company | Abbvie |
| 2 of 8 | |
|---|---|
| Drug Name | Calcitriol |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | Calcitriol Injection is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcitriol Injection is available in 1 mL ampuls. Each 1 mL contains calcit... |
| Active Ingredient | Calcitriol |
| Dosage Form | Injectable; Capsule; Solution |
| Route | Injection; Oral |
| Strength | 0.5mcg; 1mcg/ml; 0.001mg/ml; 0.25mcg; 0.002mg/ml |
| Market Status | Prescription |
| Company | Rockwell Medcl; Fresenius Kabi Usa; Teva; Fresenius Medcl; Luitpold; Sagent Pharms; Banner Pharmacaps; Roxane; Akorn |
| 3 of 8 | |
|---|---|
| Drug Name | Rocaltrol |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | Rocaltrol (calcitriol) is a synthetic vitamin D analog which is active in the regulation of the absorption of calcium from the gastrointestinal tract and its utilization in the body. Rocaltrol is available as capsules containing 0.25mcg or 0.5mcg... |
| Active Ingredient | Calcitriol |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 0.5mcg; 0.25mcg; 1mcg/ml |
| Market Status | Prescription |
| Company | Validus Pharms |
| 4 of 8 | |
|---|---|
| Drug Name | Vectical |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | VECTICAL (calcitriol) Ointment 3 mcg/g is a vitamin D analog intended for topical application to the skin. The chemical name of the active ingredient is (5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol. The structural formula is:Calcitrio... |
| Active Ingredient | Calcitriol |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 3mcg/gm |
| Market Status | Prescription |
| Company | Galderma Labs |
| 5 of 8 | |
|---|---|
| Drug Name | Vectical |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | VECTICAL (calcitriol) Ointment 3 mcg/g is a vitamin D analog intended for topical application to the skin. The chemical name of the active ingredient is (5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol. The structural formula is:Calcitrio... |
| Active Ingredient | Calcitriol |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 3mcg/gm |
| Market Status | Prescription |
| Company | Galderma Labs |
| 6 of 8 | |
|---|---|
| Drug Name | Calcijex |
| Drug Label | Calcijex (calcitriol injection) is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcijex is available in 1 mL ampuls. Each 1 mL contains calcitr... |
| Active Ingredient | Calcitriol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.001mg/ml; 0.002mg/ml |
| Market Status | Prescription |
| Company | Abbvie |
| 7 of 8 | |
|---|---|
| Drug Name | Calcitriol |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | Calcitriol Injection is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcitriol Injection is available in 1 mL ampuls. Each 1 mL contains calcit... |
| Active Ingredient | Calcitriol |
| Dosage Form | Injectable; Capsule; Solution |
| Route | Injection; Oral |
| Strength | 0.5mcg; 1mcg/ml; 0.001mg/ml; 0.25mcg; 0.002mg/ml |
| Market Status | Prescription |
| Company | Rockwell Medcl; Fresenius Kabi Usa; Teva; Fresenius Medcl; Luitpold; Sagent Pharms; Banner Pharmacaps; Roxane; Akorn |
| 8 of 8 | |
|---|---|
| Drug Name | Rocaltrol |
| PubMed Health | Calcitriol |
| Drug Classes | Antipsoriatic, Nutritive Agent |
| Drug Label | Rocaltrol (calcitriol) is a synthetic vitamin D analog which is active in the regulation of the absorption of calcium from the gastrointestinal tract and its utilization in the body. Rocaltrol is available as capsules containing 0.25mcg or 0.5mcg... |
| Active Ingredient | Calcitriol |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 0.5mcg; 0.25mcg; 1mcg/ml |
| Market Status | Prescription |
| Company | Validus Pharms |
Bone Density Conservation Agents; Calcium Channel Agonist; Vitamins
National Library of Medicine, SIS; ChemIDplus Record for 1, 25-Dihydroxycholecalciferol (32222-06-3), MESH Heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Calcium Channel Agonists; Dermatologic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Medication: Calcium regulator; vitamin (antirachitic)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 277
Therapeutic doses of specific vitamin D analogs are used in the treatment of chronic hypocalcemia, hypophosphatemia, rickets, and osteodystrophy associated with various medical conditions including chronic renal failure, familial hypophosphatemia, and hypoparathyroidism (postsurgical or idiopathic, or pseudohypoparathyroidism). Some analogs have been found to reduct elevated parathyroid hormone concentrations in patients with renal osteodystrophy associated with hyperparathyroidism. Theoretically, any of the vitamin D analogs may be used for the above conditions, However, because of their pharmacologic properties, some may be more useful in certain situations than others. Alfacalcidol, calcitriol, and dihydrotachysterol are usually preferred in patients with renal failure since these patients have impaired ability to synthesize calcitriol from cholecalciferol and ergocalciferol; therefore, the response is more predictable. In addition, their shorter half-lives may make toxicity easier to manage (hypercalcemia reverses more quickly). Ergocalciferol may not be the preferred agent in the treatment of familial hypophosphatemia or hypoparathyroidism because the large doses needed are associated with a risk of overdose and hypercalcemia; dihydrotachysterol and calcitriol may be preferred. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2966
For more Therapeutic Uses (Complete) data for 1,25-DIHYDROXYCHOLECALCIFEROL (6 total), please visit the HSDB record page.
... When serum alkaline phosphate decreases, serum calcium rises. Metastatic calcification, decrease in renal function, and increased serum phosphate levels are possible consequences.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 1216
POTENTIAL ADVERSE EFFECTS ON FETUS: Teratogenic in animals at high doses (4-15x recommended human dose). In humans, maternal hypercalcemia during pregnancy may increase fetal sensitivity to effects of vitamin D, suppression of parathyroid function or a syndrome of elfin facies, mental retardation, and congenital supravalvular aortic stenosis. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: No known problems at recommended daily allowance. /Cholecalciferol from table II/
Stockton, D.L. and A.S. Paller. J Am Acad Dermatol 23 (1):87-103 (1990)
Doses of vitamin D analogs that do not exceed the physiologic requirement are usually nontoxic. However, some infants and patients with sarcoidosis or hypoparathyroidism may have increased sensitivity to vitamin D analogs. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Decreased renal function without hypercalcemia has also been reported in patients with hypoparathyroidism after long-term vitamin D analog therapy. Before therapy with vitamin D analogs is initiated, serum phosphate concentrations must be controlled. To avoid ectopic calcification, the serum calcium (in mg/dL) times phosphorus (in mg/dL) should not be allowed to exceed 70. Because administration of vitamin D analogs may increase phosphate absorption, patients with renal failure may require adjustment in the dosage of aluminum-containing antacids used to decrease phosphate absorption. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
For more Drug Warnings (Complete) data for 1,25-DIHYDROXYCHOLECALCIFEROL (13 total), please visit the HSDB record page.
Used to treat vitamin D deficiency or insufficiency, refractory rickets (vitamin D resistant rickets), familial hypophosphatemia and hypoparathyroidism, and in the management of hypocalcemia and renal osteodystrophy in patients with chronic renal failure undergoing dialysis. Also used in conjunction with calcium in the management and prevention of primary or corticosteroid-induced osteoporosis.
FDA Label
Calcitriol is a biologically active calcitrophic hormone with anti-osteoporotic, immunomodulatory, anticarcinogenic, antipsoriatic, antioxidant, and mood-modulatory activities. Its main sites of action are the intestine, bone, kidney and parathyroid hormone. Calcitriol is a ligand for the vitamin D nuclear receptor, which is expressed in, but not limited to, gastrointestinal (GI) tissues, bones, and kidneys. As an active form of vitamin D3, calcitriol elevates the plasma levels of calcium by stimulating intestinal calcium uptake, increasing reabsorption of calcium by the kidneys, and possibly increasing the release of calcium from skeletal stores. The duration of pharmacologic activity of a single dose of exogenous calcitriol is expected to be about 3 to 5 days. In addition to its important role in calcium metabolism, other pharmacological effects of calcitriol have been studied in various conditions including cancer models. Various studies demonstrated expression of vitamin D receptors in cancer cell lines, including mouse myeloid leukemia cells. Calcitriol has been found to induce differentiation and/or inhibit cell proliferation _in vitro_ and _in vivo_ in many cell types, such as malignant cell lines carcinomas of the breast, prostate, colon, skin, and brain, myeloid leukemia cells, and others. In early human prostate cancer trials, administration of 1.5 g/d calcitriol in male participants resulted in a reduction in the rate of PSA rise in most participants, however it was coincided with dose-limiting hypercalcemia in most participants. Hypercalcemia and hypercalcuria were evident in numerous initial trials, and this may be due to these trials not testing the drug at concentrations that are active in preclinical systems. Findings from preclinical data show an additive or synergistic antineoplastic action of calcitriol when combined with agents including dexamethasone, retinoids, and radiation, as well as several cytotoxic chemotherapy drugs such as platinum compounds. Vitamin D deficiency has long been suspected to increase the susceptibility to tuberculosis. The active form of calcitriol, 1,25-(OH)2-D3, has been found to enhance the ability of mononuclear phagocytes to suppress the intracellular growth of Mycobacterium tuberculosis. 1,25-(OH)2-D3 has demonstrated beneficial effects in animal models of such autoimmune diseases as rheumatoid arthritis. Vitamin D appears to demonstrate both immune-enhancing and immunosuppressive effects.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Calcium-Regulating Hormones and Agents
Hormones and molecules with calcium-regulating hormone-like actions that modulate OSTEOLYSIS and other extra-skeletal activities to maintain calcium homeostasis. (See all compounds classified as Calcium-Regulating Hormones and Agents.)
Calcium Channel Agonists
Agents that increase calcium influx into calcium channels of excitable tissues. This causes vasoconstriction in VASCULAR SMOOTH MUSCLE and/or CARDIAC MUSCLE cells as well as stimulation of insulin release from pancreatic islets. Therefore, tissue-selective calcium agonists have the potential to combat cardiac failure and endocrinological disorders. They have been used primarily in experimental studies in cell and tissue culture. (See all compounds classified as Calcium Channel Agonists.)
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CC - Vitamin d and analogues
A11CC04 - Calcitriol
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AX - Other antipsoriatics for topical use
D05AX03 - Calcitriol
Absorption
Upon administration, calcitriol is rapidly absorbed from the intestines. When a single oral dose of 0.5 mcg of calcitriol was administered, the mean serum concentrations of calcitriol rose from a baseline value of 40.04.4 (SD) pg/mL to 60.04.4 pg/mL at 2 hours, and declined to 53.06.9 at 4 hours, 507.0 at 8 hours, 444.6 at 12 hours and 41.55.1 at 24 hours. Following administration of single doses of 0.25 to 1.0 mcg of calcitriol, the peak plasma concentrations were reached within 3 to 6 hours. In a pharmacokinetic study, the oral bioavailability was 70.65.8% in healthy male volunteers and 72.24.8% in male patients with uraemia.
Route of Elimination
In normal subjects, approximately 27% and 7% of the radioactivity appeared in the feces and urine, respectively, within 24 hours. Calcitriol undergoes enterohepatic recycling and biliary excretion. The metabolites of calcitriol are excreted primarily in feces. Cumulative excretion of radioactivity on the sixth day following intravenous administration of radiolabeled calcitriol averaged 16% in urine and 49% in feces.
Volume of Distribution
Upon intravenous administration, the volume of distribution of calcitriol was 0.490.14 L/kg in healthy male volunteers and 0.270.06l/kg in uraemic male patients participating in a pharmacokinetic study. There is some evidence that calcitriol is transferred into human milk at low levels (ie, 2.20.1 pg/mL) in mothers. Calcitriol from maternal circulation may also enter the fetal circulation.
Clearance
The metabolic clearance rate was 23.54.34 ml/min in healthy male volunteers and 10.11.35 ml/min in male patients with uraemia. In the pediatric patients undergoing peritoneal dialysis receiving dose of 10.2 ng/kg (SD 5.5 ng/kg) for 2 months, the clearance rate was 15.3 mL/hr/kg.
Many vitamin D analogs are readily absorbed from the GI tract following oral administration if fat absorption is normal. The presence of bile is required for absorption of ergocalciferol and the extent of GI absorption may be decreased in patients with hepatic, biliary, or GI disease (e.g., Crohn's disease, Whipple's disease, sprue). Because vitamin D is fat soluble, it is incorporated into chylomicrons and absorbed via the lymphatic system; approximately 80% of ingested vitamin D appears to be absorbed systemically through this mechanism, principally in the small intestine. Although some evidence suggested that intestinal absorption of vitamin D may be decreased in geriatric adults, other evidence did not show clinically important age-related alterations in GI absorption of the vitamin in therapeutic doses. It currently is not known whether aging alters the GI absorption of physiologic amounts of vitamin D. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
After oral administration of calcitriol, there is about a 2-hour lag-time before calcium absorption in the GI tract increases. Maximal hypercalcemic effect occurs in about 10 hours, and the duration of action of calcitriol is 3-5 days.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
Time to peak serum concentration: Oral: Approximately 3 to 6 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
The primary route of excretion of vitamin D is the bile; only a small percentage of an administered dose is found in urine. /Vitamin D/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1730
For more Absorption, Distribution and Excretion (Complete) data for 1,25-DIHYDROXYCHOLECALCIFEROL (10 total), please visit the HSDB record page.
Metabolism of calcitriol involves two pathways. The first pathway involves 24-hydroxylase activity in the kidney; this enzyme is also present in many target tissues which possess the vitamin D receptor such as the intestine. The end product of this pathway is a side chain shortened metabolite, calcitroic acid. The second pathway involves the conversion of calcitriol via the stepwise hydroxylation of carbon-26 and carbon-23, and cyclization to yield ultimately 1a,25R(OH)2-26,23S-lactone D3, which appears to be the major metabolite circulating in humans. Ohter identified metabolites of calcitriol include 1, 25(OH)2-24-oxo-D3; 1, 23,25(OH)3-24-oxo-D3; 1, 24R,25(OH)3D3; 1, 25S,26(OH)3D3; 1, 25(OH)2-23-oxo-D3; 1, 25R,26(OH)3-23-oxo-D3 and 1, (OH)24,25,26,27-tetranor-COOH-D3.
Calcitriol is the active form of vitamin D3 (cholecalciferol). The natural or endogenous supply of vitamin D in man mainly depends on ultraviolet light for conversion of 7-dehydrocholesterol to vitamin D3 in the skin. Vitamin D3 must be metabolically activated in the liver and the kidney before it is fully active on its target tissues. The initial transformation is catalyzed by a vitamin D3-25-hydroxylase enzyme present in the liver, and the product of this reaction is 25-(OH)D3 (calcifediol). The latter undergoes hydroxylation in the mitochondria of kidney tissue, and this reaction is activated by the renal 25-hydroxyvitamin D3-1-a-hydroxylase to produce 1,25-(OH)2D3 (calcitriol), the active form of vitamin D3.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 411
1,25-Dihydroxycholecalciferol (calcitriol) and 1,25-dihydroxyergocalciferol appear to be metabolized to their respective trihydroxy metabolites (i.e., 1,24,25-trihydroxycholecalciferol, 1,24,25-trihydroxyergocalciferol) and to other compounds. The principal metabolite excreted in urine is calcitroic acid, which is more water soluble. Although all the metabolites of cholecalciferol and ergocalciferol have not been identified, hepatic microsomal enzymes may be involved in degrading metabolites of ergocalciferol and cholecalciferol.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
Calcitriol /(1,25-dihydroxy-vitamin D)/ is hydroxylated to 1,24,25-(OH)3-D by a renal hydroxylase that is induced by calcitriol and suppressed by those factors that stimulate the 25-OHD-1-alpha-hydroxylase. This enzyme also hydroxylates 25-OHD to form 24,25-(OH)2D. Both 24-hydroxylated compounds are less active than calcitriol and presumably represent metabolites destined for excretion. Side chain oxidation of calcitriol also occurs.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1730
To evaluate the relation between daily and fasting urinary calcium excretion and serum 1,25-dihydroxyvitamin D (II) concentrations, 6 healthy men were studied during control and during chronic oral calcitrol (I) administration (0.6, 1.2, or 1.8 nmols every 6 hours for 6-12 days) while they ate normal and low calcium diets (19.2 or 4.2 mmols Ca/day). Daily urinary calcium excretion was directly related to serum II concentrations, but increased more while subjects ate the normal calcium diet than when eating the low calcium diet. During I and ingestion of the low calcium diet, daily urinary calcium excretion averaged 7.32 mmole/day, exceeding the dietary calcium intake. Fasting urinary calcium/creatinine exceeded 0.34 mmol/mmol (the upper limit of normal) on either diet. When serum II concentrations are elevated, a high fasting urinary calcium/creatinine or high daily urinary calcium excretion, even on a low calcium diet, is insufficient criteria for the documentation of a renal calcium leak.
ADAMS ND ET AL; EFFECTS OF CALCITROL ADMINISTRATION ON CALCIUM METABOLISM IN HEALTHY MEN; KIDNEY INT 21(1) 90 (1982)
For more Metabolism/Metabolites (Complete) data for 1,25-DIHYDROXYCHOLECALCIFEROL (7 total), please visit the HSDB record page.
After administration of single oral doses, the elimination half life was 5-8 hours.
Plasma half-life: 3 to 6 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
The mechanism of action of calcitriol in the treatment of psoriasis is accounted for by their antiproliferative activity for keratinocytes and their stimulation of epidermal cell differentiation. The anticarcinogenic activity of the active form of Calcitriol appears to be correlated with cellular vitamin D receptor (VDR) levels. Vitamin D receptors belong to the superfamily of steroid-hormone zinc-finger receptors. VDRs selectively bind 1,25-(OH)2-D3 and retinoic acid X receptor (RXR) to form a heterodimeric complex that interacts with specific DNA sequences known as vitamin D-responsive elements. VDRs are ligand-activated transcription factors. The receptors activate or repress the transcription of target genes upon binding their respective ligands. It is thought that the anticarcinogenic effect of Calcitriol is mediated via VDRs in cancer cells. The immunomodulatory activity of calcitriol is thought to be mediated by vitamin D receptors (VDRs) which are expressed constitutively in monocytes but induced upon activation of T and B lymphocytes. 1,25-(OH)2-D3 has also been found to enhance the activity of some vitamin D-receptor positive immune cells and to enhance the sensitivity of certain target cells to various cytokines secreted by immune cells. A study suggests that calcitriol plays an immunoregulatry role by suppressing the aryl hydrocarbon receptor (AhR) expression in human Th9, a pro-inflammatory CD4 T cell subset. This suppression subsequently leads to repressed expression of BATF, a transcription factor essential for Th9. Calcitriol has also been found to induce monocyte differentiation and to inhibit lymphocyte proliferation and production of cytokines, including interleukin IL-1 and IL-2, as well as to suppress immunoglobulin secretion by B lymphocytes.
Ergocalciferol and doxercalciferol (1-hydroxyergocalciferol); cholecalciferol and calcifediol (25-hydroxycholecalciferol); and dihydrotachysterol in their activated forms (1,25-dihydroxyergocalciferol; 1,25-dihydroxycholecalciferol [calcitriol]; and 25-hydroxydihydrotachysterol; respectively), along with parathyroid hormone and calcitonin, regulate serum calcium concentrations; in addition to conversion to the active 1,25-dihydroxycholecalciferol, calcifediol also has intrinsic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
Calcitriol (activated vitamin D) enhances the efficiency of intestinal calcium absorption along the entire small intestine, but principally in the duodenum and jejunum. Calcitriol also enhances phosphorus absorption along the entire small intestine, but principally in the jejunum and ileum. The activated forms of ergocalciferol, doxercalciferol, and cholecalciferol may have a negative feedback effect on parathyroid hormone (PTH) production.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
Calcitriol appears to act in intestine in manner that is analogous to the way steroid hormones such as estrogens act on target tissues. ... Cytosol of chicken intestinal cells contains a 3.7 S protein that binds calcitriol specifically and with high affinity. Formation of complex with this receptor facilitates transfer of calcitriol to nuclear chromatin. ... Calcitriol stimulates synthesis of RNA and at least two proteins in intestinal mucosa, alkaline phosphatase and a calcium-binding protein. ... It was proposed that the calcium-binding protein is involved in transport of calcium. ... /However/, it has been reported that calcitriol-induced stimulation of intestinal transport of phosphate precedes that of calcium, and it is possible that primary effect of the vitamin is on phosphate rather than calcium transport.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1541
The effects of 1,25-dihydroxyvitamin D3 (I) on the human promyelocytic leukemia cell line HL-60 were investigated. I induces the differentiation of HL-60 into mono- and multinucleated macrophage-like cells. Phenotypic change is evident within 24 hours and reaches a plateau at 72-96 hours of incubation. The changes are metabolite-specific and include adherence to substrate, acquisition of the morphological features of mature monocytes, a 4 to 6-fold enhancement in lysozyme synthesis and secretion, increase in the fraction of alpha-naphthyl acetate monocyte-associated cell surface antigens. Treated HL-60 cells acquire the capacity to bind and degrade bone matrix, 2 of the essential functional characteristics of osteoclasts and related bone-resorbing cells. Evidently, vitamin D3 enhances bone resorption and osteoclastogenesis in vivo by promoting the differentiation of precursor cells.
BAR-SHAVIT Z ET AL; INDUCTION OF MONOCYTIC DIFFERENTIATION AND BONE RESORPTION BY 1,25-DIHYDROXYVITAMIN D3; PROC NATL ACAD SCI USA 80(19) 5907 (1983)
For more Mechanism of Action (Complete) data for 1,25-DIHYDROXYCHOLECALCIFEROL (6 total), please visit the HSDB record page.








