



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
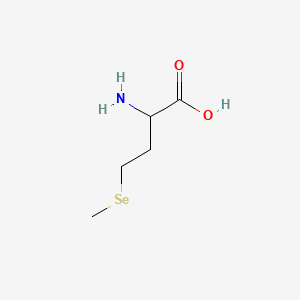

1. Butanoic Acid, 2-amino-4-(methylseleno)-
2. Radioselenomethionine
3. Se 75, Selenomethionine
4. Selenomethionine Hydrochloride, (s)-isomer
5. Selenomethionine Se 75
6. Selenomethionine, (+,-)-isomer
7. Selenomethionine, (r)-isomer
8. Selenomethionine, (s)-isomer
9. Sethotope
1. Dl-selenomethionine
2. 1464-42-2
3. Seleno-dl-methionine
4. Butanoic Acid, 2-amino-4-(methylseleno)-
5. 2578-28-1
6. (+-)-selenomethionine
7. 2-amino-4-(methylselanyl)butanoic Acid
8. Methionine, Seleno
9. Selenium Methionine
10. 2-amino-4-methylselanylbutanoic Acid
11. Selenomethionine (van)
12. 2-amino-4-(methylselenyl)butyric Acid
13. Butyric Acid, 2-amino-4-(methylselenyl)-
14. 2-amino-4-(methylseleno)butanoic Acid
15. 2-amino-4-(methylseleno)butyric Acid
16. J9v40v4pkz
17. Selenomethionine [usan]
18. (r)-2-amino-4-(methylselanyl)butanoic Acid
19. Chebi:27585
20. Ncgc00159438-02
21. Seleno-dl-methionine;dl-selenomethionine
22. L-(+)-selenomethionine
23. Ccris 3970
24. Hsdb 3564
25. Einecs 215-977-0
26. Unii-j9v40v4pkz
27. Mfcd00063089
28. Dl-se-met
29. 2-amino-4-methylselanyl-butanoic Acid
30. (s)-2-amino-4-(methylselenyl)butyric Acid
31. Bmse000291
32. Dsstox_cid_20609
33. Dsstox_rid_79510
34. Dsstox_gsid_40609
35. Schembl63321
36. Selenomethionine, Dl-
37. Selenomethionine Dl-form
38. (+/-)-selenomethionine
39. Chembl1474517
40. Dtxsid7040609
41. Dl-selenomethionine [hsdb]
42. Bcp24111
43. Hy-b1000
44. Tox21_111668
45. Dl-selenomethionine [who-dd]
46. Nsc172801
47. Nsc724226
48. 2-amino-4-(methylseleno)-butanoicaci
49. Selenomethionine Dl-form [mi]
50. Akos015854582
51. Cs-4494
52. Nsc-172801
53. Nsc-724226
54. Seleno-dl-methionine, >=99% (tlc)
55. Ncgc00159438-03
56. Cas-1464-42-2
57. Ft-0625510
58. Ft-0628011
59. Ft-0700913
60. D92258
61. A818011
62. Q415925
63. Seleno-dl-methionine, Certified Reference Material, Tracecert(r)
| Molecular Weight | 196.12 g/mol |
|---|---|
| Molecular Formula | C5H11NO2Se |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 196.99550 g/mol |
| Monoisotopic Mass | 196.99550 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 97 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Radioactive form of /selenomethionine/ as diagnostic aid (pancreas function determination); radioactive agent.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1457
Available forms of /nutritional/ supplements include high selenium yeast, L-selenomethionine, sodium selenate and sodium selenite. /L-selenomethionine/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 568
/Experimental Therapy/ ... Squamous dysplasia, the accepted histological precursor for esophageal squamous cell carcinoma, represents a potentially modifiable intermediate end point for chemoprevention trials in high-risk populations. ...Aa randomized, controlled trial of selenomethionine 200 ug daily and/or celecoxib 200 mg twice daily (2 x 2 factorial design) among residents of Linxian, People's Republic of China. Subjects had histologically confirmed mild or moderate esophageal squamous dysplasia at baseline. Esophagogastroduodenoscopy was performed before and after a 10-month intervention. Per-subject change (regression, stable, or progression) in the worst dysplasia grade was defined as the primary end point. Results were compared by agent group (selenomethionine vs placebo; celecoxib vs placebo). ... Two hundred sixty-seven subjects fulfilled all eligibility criteria, and 238 (89%) completed the trial. Overall, selenomethionine resulted in a trend toward increased dysplasia regression (43% vs 32%) and decreased dysplasia progression (14% vs 19%) compared with no selenomethionine (P = .08). In unplanned stratified analyses, selenomethionine favorably affected a change in dysplasia grade among 115 subjects with mild esophageal squamous dysplasia at baseline (P = .02), but not among 123 subjects with moderate esophageal squamous dysplasia at baseline (P = 1.00). Celecoxib status did not influence changes in dysplasia grade overall (P = .78) or by baseline histology subgroup. ... After a 10-month intervention, neither selenomethionine nor celecoxib inhibited esophageal squamous carcinogenesis for all high-risk subjects. However, among subjects with mild esophageal squamous dysplasia at baseline, selenomethionine did have a protective effect...
PMID:16143126 Limburg PJ et al; Gastroenterology 129 (3): 863-73 (2005)
/Experimental Therapy/ ... Studies were carried out on nude mice bearing human colorectal carcinoma SW480 cell line xenografts to evaluate the chemotherapeutic potential of selenium containing compounds such as sodium selenite (SSe) and selenomethionine (SeMet). Three doses of anticancer drugs were used, including 0.1 mg/kg/day SSe (LSSe), 2 mg/kg/day SSe (HSSe), and 2 mg/kg/day SeMet ... administered by IP injection for 21 days. ... The pathologic changes and the cell apoptosis in tumor tissue /were observed/ by HE staining and TUNNEL assay after HSSe and SeMet treatment. GSH level and antioxidant enzyme GPX activity in tumor tissues were assessed. In addition, Western blotting was used to detect the expression of apoptosis-related proteins. The results suggested that HSSe and SeMet had significantly inhibited tumor growth in vivo. ... GSH level was a bit increased but the GPX activity was reduced. Moreover, SSe and SeMet treatment downregulated the expression of the protein Bcl-xL, increased the expression of Bax, Bad, and Bim, and activated caspase-9. SSe and SeMet may be the selective, low-toxic anticancer agents to treat human colorectal carcinoma cancer.
PMID:19911698 Yang Y et al; Oncol Res 18 (1): 1-8 (2009)
/Experimental Therapy/ ... Based on clinical findings and recent studies in selenoprotein gene-modified mice, it is likely that the antioxidant function of one or more selenoproteins is responsible for the chemopreventive effect of Se. Furthermore, upregulation of phase 2 enzymes by Se has been implicated as a possible chemopreventive mechanism at supranutritional dietary levels. Se-methylselenocysteine (SeMSC), a naturally occurring organic Se product, is considered as one of the most effective chemopreventive selenocompounds...
PMID:17728283 Zhang J et al; Toxicol Sci 101 (1): 22-31 (2007)
The ability of selenomethionine (SeMet) to be incorporated into the body proteins in place of methionine (Met) furthermore provides a means of reversible Se storage in organs and tissues. This property is not shared by any other naturally occurring selenoamino acid and thus could be associated with a specific physiological function of SeMet. Since higher animals cannot synthesize SeMet, yet from it all needed forms of Se are produced, SeMet meets the criteria of an essential amino acid. Accordingly, SeMet, or enriched food sources thereof, are appropriate forms of Se for human nutritional Se supplementation. However, while SeMet or Se yeast are already widely used in over-the-counter nutritional supplements, infant formulas and parenteral feeding mixtures still contain Se in the form of sodium selenate or sodium selenite, even though these are not the normal nutritional forms of Se. In animal nutrition, these inorganic selenium salts are increasingly replaced by food sources of SeMet such as Se yeast. Synthetic SeMet could also be employed as a feed additive, but its regulatory status is as yet undetermined. The optimal nutritional levels of SeMet for different animal species still need to be determined. The expectation is that lower additions to feedstock of equivalent levels of SeMet will suffice to achieve adequacy than currently approved maximum levels of Se in the form of inorganic Se salts.
PMID:14639782 Schrauzer GN; Adv Food Nutr Res 47: 73-112 (2003)
SELECT stands for the Selenium and Vitamin E Cancer Prevention Trial, a clinical trial to see if one or both of these substances prevent prostate cancer when taken as dietary supplements. ... Enrollment for the trial began in 2001 and ended in 2004. More than 400 sites in the United States, Puerto Rico, and Canada took part in the study. Over 35,000 men participated in SELECT. SELECT was initially planned for a follow-up of a minimum of seven years and a maximum of 12 years. However, the independent Data and Safety Monitoring Committee (DSMC) for the trial met on September 15, 2008, to review SELECT study data and found that selenium and vitamin E, taken alone or together did not prevent prostate cancer. They also determined that it was unlikely selenium and vitamin E supplementation would ever produce a 25 percent reduction in prostate cancer, as the study was designed to show. As a result, SELECT participants were told in October 2008 to stop taking their study supplements. Although there were no statistically significant differences (in other words, these differences could have occurred by chance alone) in the rates of prostate cancer between the four groups in the trial, there was a larger number of cases in men taking only vitamin E. The difference does not prove that vitamin E causes prostate cancer and may be due to chance. ... deaths combined, or the overall incidence of cardiovascular events between the study groups. Based on data reported from the beginning of the trial, there were more new cases of diabetes in men taking only selenium (10 percent of these men) as compared to the men taking placebo (9.3 percent). This finding was not statistically significant, does not prove an increased risk from selenium and may be due to chance. /Selenium containing preparations/
National Cancer Institute; Selenium and Vitamin E Cancer Prevention Trial (SELECT) (February 3, 2010). Available from, as of March 30, 2011: https://www.cancer.gov/newscenter/qa/2008/selectqa
The most frequently reported adverse reactions of selenosis or chronic selenium toxicity are hair and nail brittleness and loss. Other symptoms include skin rash, garlic-like breath odor, fatigue, irritability and nausea and vomiting. /Selenium containing preparations/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 567
Intakes of selenium less than 900 ug daily (for adults) are unlikely to cause adverse reactions. Prolonged intakes of selenium of doses of 1,000 ug (or 1 mg) or greater daily may cause adverse reactions. /Selenium containing preparations/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 567
Pregnant women and nursing mothers should avoid selenium intakes greater than Recommended Dietary Allowance amounts. /Selenium containing preparations/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 567
... Lambs were orally administered a single dose of selenium as either sodium selenite or selenomethionine and were monitored for 7 days, after which they were euthanized and necropsied. ... Analysis of liver, kidney cortex, heart, blood, and serum revealed linear, dose-dependent increases in selenium concentration. However, tissue selenium concentration in selenomethionine-treated lambs were significantly greater than that in lambs treated with equivalent doses of sodium selenite.
PMID:16566258 Tiwary AK et al; J Vet Diagn Invest. 2006 Jan;18(1):61-70 (2006)
Se-Methylated selenoamino acids, Se-methylselenocysteine (MeSeCys) and selenomethionine (SeMet), are chemically inert storage forms of selenium in selenium-accumulators, and a nutritional and supplemental source. ... Male Wistar rats were depleted of endogenous natural abundance selenium with a single (80)Se-enriched isotope, and then (76)Se-MeSeCys, (77)Se-SeMet and (82)Se-selenite were orally administered simultaneously at 25 ug Se/kg body weight each. Organs and body fluids were obtained at 3, 6, 9 and 12 hr, and 1 and 2 days later, and subjected to speciation analysis. The main characteristics of the metabolism were as follows; MeSeCys was incorporated into selenoprotein P slightly more than or at a comparable level to that of SeMet but less than that of selenite. MeSeCys and SeMet but not selenite was taken up by organs in their intact forms. MeSeCys and SeMet were delivered specifically to the pancreas and present in a form bound to an identical or similar protein. Trimethylselenonium (TMSe) was only produced from MeSeCys, i.e., not from SeMet or selenite, in the kidneys. Both selenosugars A and B of MeSeCys, SeMet and selenite origin were detected in the liver but only selenosugar B in the kidneys...
PMID:17056079 Suzuki KT et al; Toxicol Appl Pharmacol 217 (2): 185-95 (2006)
... Rats were depleted of endogenous natural abundance selenium by feeding a single selenium stable isotope ((82)Se-selenite) and then administered (76)Se-selenite and (77)Se-selenomethionine ((77)Se-SeMet)simultaneously. Biological samples were subjected to quantification and speciation analysis by HPLC-ICPMS. Metabolites of the labeled (76)Se and (77)Se and interaction with endogenous selenium were traced and examined without interference from the corresponding endogenous natural abundance isotopes. Differences in the distribution and metabolism among organs and between the two nutritional selenocompounds were compared under exactly identical biological and analytical conditions: (1) selenite was distributed more efficiently than SeMet in organs and body fluids except the pancreas. (2) SeMet was taken up by organs in its intact form. (3) Selenium of SeMet origin was distributed selectively in the pancreas and mostly bound to a protein together with intact SeMet. (4) Selenosugars A and B but not trimethylselenonium (TMSe) were detected in the liver. (5) Selenosugar B and TMSe were detected in the kidneys.
PMID:16956638 Suzuki KT et al; Toxicol Appl Pharmacol 217 (1): 43-50 (2006)
... More than 80% of orally administered selenomethionine ... is absorbed by rats.
Norderg, G.F. et al; Handbook on the Toxicology of Metals 3rd ed. Academic Press, Burlington, MA. 2007, p. 789
For more Absorption, Distribution and Excretion (Complete) data for SELENIUM METHIONINE (15 total), please visit the HSDB record page.
Most dietary selenium is in the form of selenomethionine ... or selenocysteine, both of which are well absorbed. Other forms of selenium include selenate and selenite, which are not major dietary constituents, but are commonly used in fortified foods and dietary supplements. Two pools of reserve selenium are present in the body. The first is as selenomethionine, which is not known to have a physiological function separate from that of methionine. The second reserve pool is the selenium found in liver glutathione peroxidase. Ingested selenite, selenate, and selenocysteine are all metabolized directly to selenide, the reduced form of selenium. Selenomethionine can also be metabolized to selenide.
National Academies of Science, Institute of Medicine; "Selenium" p.380-7 in Dietary Reference Intakes. The Essential Guide to Nutrient Requirements ed. by J.J. Otten et al., Washington, DC, National Academies Press (2006)
To obtain quantitative information on human metabolism of selenium, ... selenium speciation analysis /was performed/ by HPLC/ICPMS on samples of human urine from one volunteer over a 48-hour period after ingestion of selenium (1.0 mg) as sodium selenite, L-selenomethionine, or DL-selenomethionine. The three separate experiments were performed in duplicate. Normal background urine from the volunteer contained total selenium concentrations of 8-30 ug Se/L (n=22) but ... only about 30-70% could be quantified by HPLC/ICPMS. The major species in background urine were two selenosugars, namely methyl-2-acetamido-2-deoxy-1-seleno-beta-D-galactopyranoside (selenosugar 1) and its deacylated analog methyl-2-amino-2-deoxy-1-seleno-beta-D-galactopyranoside (selenosugar 3). Selenium was rapidly excreted after ingestion of the selenium compounds: the peak concentrations (approximately 250-400 ug Se/L, normalized concentrations) were recorded within 5-9 hours, and concentrations had returned to close to background levels within 48 hours, by which time 25-40% of the ingested selenium, depending on the species ingested, had been accounted for in the urine. In all experiments, the major metabolite was selenosugar 1, constituting either approximately 80% of the total selenium excreted over the first 24 hours after ingestion of selenite or L-selenomethionine or approximately 65% after ingestion of DL-selenomethionine. Selenite was not present at significant levels (<1 ug Se/L) in any of the samples; selenomethionine was present in only trace amounts (approximately 1 ug/L, equivalent to less than 0.5% of the total Se) following ingestion of L-selenomethionine, but it constituted about 20% of the excreted selenium (first 24 hours) after ingestion of DL-selenomethionine, presumably because the D form was not efficiently metabolized. Trimethylselenonium ion, a commonly reported urine metabolite, could not be detected (<1 ug/L) in the urine samples after ingestion of selenite or selenomethionine. Cytotoxicity studies on selenosugar 1 and its glucosamine isomer (selenosugar 2, methyl-2-acetamido-2-deoxy-1-seleno-beta-D-glucosopyranoside) were performed with HepG2 cells derived from human hepatocarcinoma, and these showed that both compounds had low toxicity (about 1000-fold less toxic than sodium selenite). The results support earlier studies showing that selenosugar 1 is the major urinary metabolite after increased selenium intake, and they suggest that previously accepted pathways for human metabolism of selenium involving trimethylselenonium ion as the excretionary end product may need to be re-evaluated.
PMID:1613213 Kuehnelt D et al; Anal Bioanal Chem 383 (2): 235-46 (2005)
When selenium is consumed as selenomethionine or other organic forms that occur naturally in foods, it is released as selenite by postabsorptive catabolism.
Olson et al; Phytochem 9: 1181-88 (1970)
A major source of selenium in the diet is selenomethionine. Once ingested by an animal, it enters the methionine pool and is not distinguished from methionine. Thus, much of the selenium in animal tissues is selenomethionine that is non-specifically incorporated in proteins at methionine positions. When selenomethionine is catabolized, its selenium becomes available to the selenium metabolic.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 646 (2005)
For more Metabolism/Metabolites (Complete) data for SELENIUM METHIONINE (6 total), please visit the HSDB record page.
The long term fate of an oral dose of (75)Se selenomethionine has been studied in 4 women. Intestinal absorption amounted to 95-97% of the dose, the first 2-wk urinary excretion accounted for 6-9% & after 8 wk, the whole body retention of (75)Se decreased exponentially with a half life of 207-290 days. ... (75)Se selenomethionine was found to be more completely absorbed and had a greater body retention of (75)Se with smaller urinary and fecal losses than (75)Se from (75)Se selenite.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 426
The half life of selenium methionine is 234 days.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 2, 2nd ed. 2001. Academic Press, San Diego, California., p. 845
Selenomethionine (SeMet) is the chemical form or major component of selenium used for cancer chemoprevention in several clinical trials. However, evidence from experimental studies indicates that SeMet has weaker anticancer effects than most other forms of selenium. ... The anticancer activity of SeMet can be enhanced by methioninase (METase), indicating that SeMet metabolites are responsible for its anticancer activity. ... Wild-type p53-expressing LNCaP human prostate cancer cells were more sensitive to cotreatment with SeMet and METase than p53-null PC3 human prostate cancer cells. SeMet and METase cotreatment significantly increased levels of superoxide and apoptosis in LNCaP cells. Cotreatment with SeMet and METase resulted in increased levels of phosphorylated p53 (Ser15), total p53, Bax, and p21(Waf1) proteins. LNCaP cells treated with SeMet and METase also showed p53 translocation to mitochondria, decreased mitochondrial membrane potential, cytochrome c release into the cytosol, and activation of caspase-9. The effects of SeMet and METase were suppressed by pretreatment with a synthetic superoxide dismutase mimic or by knockdown of p53 via RNA interference. Reexpression of wild-type p53 in PC3 cells resulted in increases in superoxide production, apoptosis, and caspase-9 activity and a decrease in mitochondrial membrane potential following cotreatment with SeMet and METase. /The/ study shows that apoptosis induced by SeMet plus METase is superoxide mediated and p53 dependent via mitochondrial pathway(s). These results suggest that superoxide and p53 may play a role in cancer chemoprevention by selenium.
PMID:17172431 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1761114 Zhao R et al; Mol Cancer Ther 5 (12): 3275-84 (2006)
... The toxicity of Se is thought to arise from its ability to substitute for sulfur during the assembly of proteins. .../More/ recent studies also indicate that some forms of selenium are capable of generating oxidative stress in an in vitro test system that includes glutathione. L-Selenomethionine, the predominant form of selenium in the eggs of oviparous vertebrates, does not generate oxidative radicals in this system, but lesions consistent with oxidative stress have been identified in fish and birds with high concentrations of Se. /This study reports/ on the ability of rainbow trout embryos to transform L-selenomethionine to a form capable of producing a superoxide radical. Oxidative stress appears to be generated by methioninase enzyme activity in the embryos that liberates methylselenol from l-selenomethionine. Methylselenol redox cycles in the presence of glutathione producing superoxide and likely accounts for oxidative lesions present in fish and birds environmentally exposed to excessive loads of selenomethionine. /L-Selenomethionine/
PMID:15087158 Palace VP et al; Ecotoxicol Environ Saf 58 (1): 17-21 (2004)
Primary cultures of porcine aortic endothelial cells were used to test the potential protective effect ... against several types of oxidative stress. The stressors included exposure to hyperoxia, treatment with paraquat, and incubation in the presence of the hypoxanthine/xanthine oxidase system. This protective effect ... of selenomethionine ... is known to increase glutathione peroxidase activity. ...
PMID:3687596 Junod A F et al; Agents Actions 22 (1/2): 176-83 (1987)
High-selenium containing yeast is being evaluated in clinical trials against colon polyp recurrence. However, the molecular targets for the anticancer effects of selenium remain unclear. Previous studies by our group demonstrated that selenomethionine-induced growth arrest appears to be mediated by activation of ERK and subsequent phosphorylation of RSK and histone H3. These results suggest that selenomethionine can alter gene expression. In the present study, we have used cDNA microarrays to determine whether gene expression differences exist in HCT116 colon cancer cells treated with selenomethionine. These experiments reveal statistically significant expression changes for 50 genes. Genes we found to increase with selenomethionine treatment include KLK6, ATOX1, SGK, GJB2, DAP-1, PLAU, VIM, DPYSL2, STC2 and PXN. Conversely, genes downregulated by selenomethionine include PRKACB, LIM, DEPP, MYC, CDH5, ELF3, VSNL1, SAT and EGLN3. Further analysis of those genes using chromatin immunoprecipitation experiments showed that phosphorylated histone H3 on serine 10 bound to the GJB2 promoter (connexin 26) or the serum glucocorticoid kinase promoter is increased with selenomethionine treatment. Cells overexpressing CX26 or DAP-1 displayed a reduced number of colonies which suggests that these two genes could play a functional role in the growth inhibitory effects of selenomethionine. These data support the notion that selenomethionine-induced growth inhibition is associated with global changes in gene expression. They also demonstrate that selenomethionine can modify chromatin state to alter gene transcription.
PMID:17374985 Goulet AC et al; Cancer Biol Ther 6 (4): 494-503 (2007)






