



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0

1. Anti Worm
2. Anti-worm
3. Bantenol
4. Banworm
5. Boots Threadworm Treatment
6. Lomper
7. Madicure
8. Mebendan
9. Mebenvet
10. Pripsen Mebendazole
11. R17635
12. Sqworm
13. Sufil
14. Surfont
15. Telmin
16. Vermicol
17. Vermidil
18. Vermox
19. Wormkuur
1. 31431-39-7
2. Vermox
3. Pantelmin
4. Telmin
5. Mebenvet
6. Ovitelmin
7. Vermicidin
8. Vermirax
9. Mebenoazole
10. Bantenol
11. Mebutar
12. Lomper
13. Mbdz
14. Besantin
15. Verpanyl
16. Noverme
17. Mebendazolum
18. Methyl (5-benzoyl-1h-benzo[d]imidazol-2-yl)carbamate
19. 5-benzoyl-2-benzimidazolecarbamic Acid Methyl Ester
20. Methyl 5-benzoyl-2-benzimidazolecarbamate
21. Carbamic Acid, (5-benzoyl-1h-benzimidazol-2-yl)-, Methyl Ester
22. Methyl 5-benzoyl-2-benzimidazolylcarbamate
23. R 17,635
24. Methyl N-(6-benzoyl-1h-benzimidazol-2-yl)carbamate
25. R 17635
26. Methyl (5-benzoyl-1h-benzimidazol-2-yl)carbamate
27. Methyl 5-benzoyl Benzimidazole-2-carbamate
28. (5-benzoyl-1h-benzimidazol-2-yl)-carbamic Acid Methyl Ester
29. Mfcd00057872
30. Methyl N-(5-benzoyl-1h-benzimidazol-2-yl)carbamate
31. Zhihuanqing
32. Ccris 4479
33. Methyl N-(6-benzoyl-1h-1,3-benzodiazol-2-yl)carbamate
34. Nsc 184849
35. 2-benzimidazolecarbamic Acid, 5-benzoyl-, Methyl Ester
36. Mebendazole Polymorph C
37. Mebendazol
38. Sufil
39. Chembl685
40. Nsc-184849
41. N-2 (5-benzoyl-benzimidazole) Carbamate De Methyle
42. N-(benzoyl-5, Benzimidazolyl)-2, Carbamate De Methyle
43. Chebi:6704
44. Carbamic Acid, N-(5-benzoylbenzimidazol-2-yl)-, Methyl Ester
45. Versid
46. Methyl 6-benzoyl-1h-benzimidazol-2-ylcarbamate
47. 81g6i5v05i
48. R-17635
49. 5-benzoyl-2-benzimidazolecarbamic Acid, Methyl Ester
50. Nsc184849
51. Methyl [5-(phenylcarbonyl)-1h-benzimidazol-2-yl]carbamate
52. Ncgc00016806-01
53. (5-benzoyl-1h-benzimidazol-2-yl)carbamic Acid Methyl Ester
54. Mebex
55. Cas-31431-39-7
56. Equivurm Plus
57. Dsstox_cid_20682
58. Dsstox_rid_79538
59. Dsstox_gsid_40682
60. Mebendazol [inn-spanish]
61. Mebendazolum [inn-latin]
62. Vermox (tn)
63. Emverm
64. Smr000036734
65. Hsdb 3232
66. Sr-01000003109
67. Einecs 250-635-4
68. Methyl N-(5-benzoyl-2-benzimidazolyl)carbamate
69. Mebatreat
70. Mebendazole (jan/usp/inn)
71. Unii-81g6i5v05i
72. Equivurmp Plus
73. N-2 (5-benzoyl-benzimidazole) Carbamate De Methyle [french]
74. Mebendazole,(s)
75. N-(benzoyl-5, Benzimidazolyl)-2, Carbamate De Methyle [french]
76. V95
77. Prestwick_310
78. Mebendazole; 4030
79. Mebendazole [usan:usp:inn:ban:jan]
80. Spectrum_001298
81. Cpd000036734
82. Prestwick0_000217
83. Prestwick1_000217
84. Prestwick2_000217
85. Prestwick3_000217
86. Spectrum2_001401
87. Spectrum3_001439
88. Spectrum4_000416
89. Spectrum5_001381
90. Mebendazole [inn]
91. Mebendazole [jan]
92. Mebendazole [hsdb]
93. Mebendazole [usan]
94. Probes1_000013
95. Probes2_000149
96. Mebendazole [vandf]
97. Cambridge Id 5250893
98. Methyl [5-(benzoyl)benzimidazol-2-yl]carbamate
99. Timtec1_000869
100. Mebendazole [mart.]
101. Oprea1_278237
102. Oprea1_768530
103. Schembl15860
104. Bspbio_000233
105. Bspbio_003178
106. Cbdive_010559
107. Kbiogr_000712
108. Kbioss_001778
109. Mebendazole [usp-rs]
110. Mebendazole [who-dd]
111. Mebendazole [who-ip]
112. Mls000028491
113. Mls006011879
114. Bidd:gt0087
115. Divk1c_000751
116. Mebendazolum [who-ip]
117. Spectrum1501110
118. Spbio_001442
119. Spbio_002154
120. Bpbio1_000257
121. Methyl N-[6-(benzoyl)-1h-benzimidazol-2-yl]carbamate
122. Dtxsid4040682
123. Mebendazole [green Book]
124. Hms502f13
125. Kbio1_000751
126. Kbio2_001778
127. Kbio2_004346
128. Kbio2_006914
129. Kbio3_002398
130. Mebendazole [orange Book]
131. Mebendazole For System Suitability
132. Ninds_000751
133. 2-benzimidazolecarbamic Acid, 5-benzoyl-, Methyl Ester (8ci)
134. Hms1536h11
135. Hms1568l15
136. Hms1921f03
137. Hms2090b03
138. Hms2092b15
139. Hms2095l15
140. Hms3259b11
141. Hms3604n11
142. Hms3712l15
143. Mebendazole [ep Monograph]
144. Mebendazole [usp Impurity]
145. Pharmakon1600-01501110
146. Zinc121541
147. Mebendazole [usp Monograph]
148. Tox21_110620
149. Bbl008298
150. Bdbm50180753
151. Ccg-39628
152. Mmv003152
153. Nsc757838
154. S4610
155. Stk093862
156. Akos000539066
157. Akos015896232
158. Carbamic Acid, (5-benzoyl-1h-benzimidazol-2-yl)-, Methyl Ester (9ci)
159. Tox21_110620_1
160. Cs-3974
161. Db00643
162. Mebendazole 100 Microg/ml In Methanol
163. Nc00639
164. Nsc-757838
165. Idi1_000751
166. Mebendazole Polymorph C [usp-rs]
167. Ncgc00016806-02
168. Ncgc00016806-03
169. Ncgc00016806-04
170. Ncgc00016806-05
171. Ncgc00016806-06
172. Ncgc00016806-07
173. Ncgc00016806-08
174. Ncgc00016806-09
175. Ncgc00016806-10
176. Ncgc00016806-12
177. Ncgc00016806-13
178. Ncgc00021698-03
179. Ncgc00021698-04
180. Ncgc00021698-05
181. Ncgc00021698-06
182. Ncgc00021698-07
183. Ac-12064
184. As-12272
185. Hy-17595
186. Sy051142
187. Sbi-0051641.p002
188. Ab00052203
189. Ft-0628179
190. Ft-0628180
191. M2273
192. En300-50844
193. D00368
194. D70118
195. 5-benzoyl-2-benzimidazolylcarbamicacidmethylester
196. Ab00052203-09
197. Ab00052203_10
198. Mebendazole, Vetranal(tm), Analytical Standard
199. 431m397
200. A820852
201. Ag-205/04588045
202. Mebendazole, Analytical Standard, >=98% (hplc)
203. Methyl (6-benzoyl-1h-benzimidazol-2-yl)carbamate
204. Q422194
205. Methyl 5-benzoyl-1h-benzo[d]imidazol-2-ylcarbamate
206. Sr-01000003109-2
207. Sr-01000003109-3
208. W-106901
209. Brd-k77987382-001-01-7
210. Brd-k77987382-001-06-6
211. Brd-k77987382-001-08-2
212. Sr-01000003109-10
213. Methyl N-(5-benzoyl-1h-1,3-benzimidazol-2-yl)carbamate
214. Z234895185
215. 5-benzoyl-1h-benzimidazol-2-yl Carbamic Acid Methyl Ester;
216. Mebendazole, European Pharmacopoeia (ep) Reference Standard
217. Mebendazole, United States Pharmacopeia (usp) Reference Standard
218. Mebendazole For System Suitability, European Pharmacopoeia (ep) Reference Standard
219. Mebendazole Polymorph C, United States Pharmacopeia (usp) Reference Standard
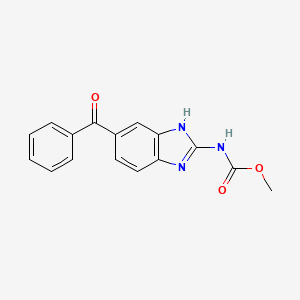
| Molecular Weight | 295.29 g/mol |
|---|---|
| Molecular Formula | C16H13N3O3 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 295.09569129 g/mol |
| Monoisotopic Mass | 295.09569129 g/mol |
| Topological Polar Surface Area | 84.1 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 423 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Mebendazole |
| PubMed Health | Mebendazole (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | Mebendazole is a (synthetic) broad-spectrum anthelmintic available as chewable tablets, each containing 100 mg of mebendazole. Inactive ingredients are: anhydrous lactose NF, corn starch, magnesium stearate, microcrystalline cellulose, sodium lauryl... |
| Active Ingredient | Mebendazole |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Mebendazole |
| PubMed Health | Mebendazole (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | Mebendazole is a (synthetic) broad-spectrum anthelmintic available as chewable tablets, each containing 100 mg of mebendazole. Inactive ingredients are: anhydrous lactose NF, corn starch, magnesium stearate, microcrystalline cellulose, sodium lauryl... |
| Active Ingredient | Mebendazole |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
Mesh Heading: Antinematodal agents
National Library of Medicine, SIS; ChemIDplus Record for Mebendazole (31431-39-7). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Mebendazole is indicated as a primary agent for tichuriasis caused by Trichuris trichiura (whipworm). /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1992
Mebendazole is indicated in the treatment of multiple intestinal roundworm infections. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1992
Mebendazole is indicated a a primary agent for enterobiasis caused by Enterobius vermicularis (pinworm). /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1992
For more Therapeutic Uses (Complete) data for MEBENDAZOLE (19 total), please visit the HSDB record page.
Organ system function (including hematopoietic and hepatic) should be assessed periodically during prolonged mebendazole therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
Other adverse effects reported rarely in patients receiving mebendazole include alopecia, rash, pruritus, urticaria, angioedema, flushing, hiccups, cough, weakness,drowsiness, chills, hypotension, seizures, transient abnormalities in liver function tests (e.g., increased serum concentrations of aminotransferases, alkaline phosphatase, and/or bilirubin), hepatitis, increased BUN, decreased hemoglobin concentration and/or hematocrit, leukopenia, thrombocytopenia, eosinophilia, hematuria, and cylindruria.Migration of roundworms through the mouth and nose also has been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
Myelosuppression manifested as neutropenia (including agranulocytosis) and/or thrombocytopenia also has been reported in patients receiving high-dose (e.g., 30-50 mg/kg daily) mebendazole therapy for extraintestinal infections; while the myelosuppression usually was reversible following discontinuance of the drug, death has occurred rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
At usual recommended dosages (i.e., 100-200 mg daily), mebendazole appears to cause minimal adverse effects. Adverse effects appear to occur more frequently when higher dosages (e.g., those used in the treatment of extraintestinal infections such as hydatid disease) are used, and may be related to effects resulting from drug-induced killing of the parasites in some cases. Transient diarrhea and abdominal pain have occurred occasionally during mebendazole treatment, but usually have been associated with massive infections and expulsion of the helminths. Nausea, vomiting, headache, tinnitus, numbness, and dizziness also have been reported occasionally during mebendazole therapy. Fever has occurred in some patients, particularly in those receiving high-dose therapy for extraintestinal infections.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
For more Drug Warnings (Complete) data for MEBENDAZOLE (9 total), please visit the HSDB record page.
For the treatment of Enterobius vermicularis (pinworm), Trichuris trichiura (whipworm), Ascaris lumbricoides (common roundworm), Ancylostoma duodenale (common hookworm), Necator americanus (American hookworm) in single or mixed infections.
Mebendazole is a (synthetic) broad-spectrum anthelmintic. The principal mode of action for Mebendazole is by its inhibitory effect on tubulin polymerization which results in the loss of cytoplasmic microtubules.
Antinematodal Agents
Substances used in the treatment or control of nematode infestations. They are used also in veterinary practice. (See all compounds classified as Antinematodal Agents.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
P02CA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02C - Antinematodal agents
P02CA - Benzimidazole derivatives
P02CA01 - Mebendazole
Absorption
Poorly absorbed (approximately 5 to 10%) from gastrointestinal tract. Fatty food increases absorption.
Route of Elimination
In man, approximately 2% of administered mebendazole is excreted in urine and the remainder in the feces as unchanged drug or a primary metabolite.
Elimination: Fecal: Approximately 95% excreted unchanged or as the primary metabolite (2-amino derivative) in feces. Renal: Approximately 2 to 5% excreted unchanged or as the primary metabolite in urine.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
Peak serum concentration: Following a dose of 100 mg twice a day for 3 days: Mebendazole: Not more than 0.03 ug/mL. 2-Amino metabolite: Not more than 0.09 ug/mL. Serum concentrations up to 0.5 ug/mL have been reported in chronic, high-dose therapy.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
Time to peak serum concentration: 2 to 5 hours (range: 0.5 to 7 hours).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
Mebendazole is highly bound to plasma proteins. It is not known if mebendazole is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
For more Absorption, Distribution and Excretion (Complete) data for MEBENDAZOLE (11 total), please visit the HSDB record page.
Primarily hepatic. Primary metabolite is 2-amino-5-benzoylbenzimidazole, but also metabolized to inactive hydroxy and hydroxyamino metabolites. All metabolites are devoid of anthelmintic activity.
Primarily hepatic; metabolized in inactive amino, hydroxy, and hydroxyamino metabolites; primary metabolite is 2-amino-5-benzoylbenzimidazole.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
Although the exact metabolic fate of mebendazole has not been fully determined, the drug is metabolized via decarboxylation to 2-amino-5(6)-benzimidazolyl phenylketone; this metabolite does not have anthelmintic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
Mebendazole ... is extensively metabolized. Two major metabolites, methyl-5-(alpha-5-hydroxybenzyl)-2-benzimidazole carbamate and 2-amino-5-benzoylbenzimidazole, have lower rates of clearance than does mebendazole itself. Mebendazole, rather than its metabolites, appears to be the active drug form. Conjugates of mebendazole and its metabolites have been found in bile, but little unchanged mebendazole appears in the urine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
2.5 to 5.5 hours (range 2.5 to 9 hours) in patients with normal hepatic function. Approximately 35 hours in patients with impaired hepatic function (cholestasis).
Normal hepatic function: 2.5 to 5.5 hours (range: 2.5 to 9 hours). Impaired hepatic function (cholestasis): Approximately 35 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
The elimination half-life of mebendazole has been reported to be about 2.8-9 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
Mebendazole causes degenerative alterations in the tegument and intestinal cells of the worm by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules. The loss of the cytoplasmic microtubules leads to impaired uptake of glucose by the larval and adult stages of the susceptible parasites, and depletes their glycogen stores. Degenerative changes in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosomes result in decreased production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth. Due to diminished energy production, the parasite is immobilized and eventually dies.
Although the exact mechanism of anthelmintic activity of mebendazole has not been fully elucidated, the drug appears to cause selective and irreversible inhibition of the uptake of glucose and other low molecular weight nutrients in susceptible helminths; inhibition of glucose uptake appears to result in endogenous depletion of glycogen stores in the helminth. Mebendazole does not inhibit glucose uptake in mammals. Mebendazole appears to cause degenerative changes in the intestine of nematodes and in the absorptive cells of cestodes. The principal anthelmintic effect of the drug appears to be degeneration of cytoplasmic microtubules within these intestinal and absorptive cells. Microtubular deterioration results in inhibition of organelle movement and interferes with the absorptive and secretory function. As a result of excessive accumulation of intracellular transport secretory granules, hydrolytic and proteolytic enzymes are released and cause cellular autolysis. This irreversible damage leads to death of the parasite.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 53
Vermicidal; may also be ovicidal for ova or most helminths; mebendazole causes degeneration of parasite's cytoplasmic microtubules and thereby selectively and irreversibly blocks glucose uptake in susceptible adult intestine-dwelling helminths and their tissue-dwelling larvae; inhibition of glucose uptake apparently results in depletion of the parasite's glycogen stores; this, in turn, results in reduced formation of adenosine triphosphate (ATP) required for survival and reproduction of the helminth; corresponding energy levels are gradually reduced until death of the parasite ensues; mebendazole does not appear to affect serum glucose concentrations in humans, however.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1993
Benzimidazoles produce many biochemical changes in susceptible nematodes, eg, inhibition of mitochondrial fumarate reductase, reduced glucose transport, and uncoupling of oxidative phosphorylation ... /but/ the primary action ... /should be/ to inhibit microtubule polymerization by binding to beta-tubulin. The selective toxicity of these agents derives from the fact that specific, high-affinity binding to parasite beta-tubulin occurs at much lower concn than does binding to the mammalian protein ... Benzimidazole-resistant Haemonchus contortus display reduced high-affinity drug binding to beta-tubulin and alterations in beta-tubulin isotype gene expression that correlate with drug resistance ... Two identified mechanisms of drug resistance in nematodes involve both a progressive loss of "susceptible" beta-tubulin gene isotypes together with emergence of a "resistant" isotype with a conserved point mutation that encodes a tyrosine instead of phenylalanine at position 200 of beta-tubulin. While this mutation may not be required for benzimidazole resistance in all parasites, eg, Giardia lamblia, benzimidazole resistance in parasitic nematodes is unlikely to be overcome by novel benzimidazole analogs, because tyrosine also is present at position 200 of human beta-tubulin. /Benzimidazoles/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126






