



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
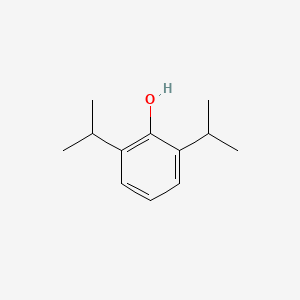

1. 2,6 Diisopropylphenol
2. 2,6-bis(1-methylethyl)phenol
3. 2,6-diisopropylphenol
4. Aquafol
5. Diprivan
6. Disoprivan
7. Disoprofol
8. Fresofol
9. Ici 35,868
10. Ici 35868
11. Ici-35,868
12. Ici-35868
13. Ici35,868
14. Ici35868
15. Ivofol
16. Propofol Abbott
17. Propofol Fresenius
18. Propofol Mct
19. Propofol Rovi
20. Propofol-lipuro
21. Recofol
1. 2,6-diisopropylphenol
2. 2078-54-8
3. Diprivan
4. Disoprofol
5. Disoprivan
6. Fresofol
7. Diisopropylphenol
8. 2,6-bis(1-methylethyl)phenol
9. Ampofol
10. Rapinovet
11. Phenol, 2,6-bis(1-methylethyl)-
12. 2,6-di(propan-2-yl)phenol
13. Propofolum
14. Recofol
15. Ivofol
16. Propofol-lipuro
17. Pofol
18. Ici 35868
19. 2,6-diisopropyl Phenol
20. Diprifusor
21. Diprofol
22. Propovan
23. Phenol, 2,6-diisopropyl-
24. Diprivan 10
25. Ici-35868
26. Lipuro
27. Nsc 5105
28. Ici 35,868
29. 2,6-bis(propan-2-yl)phenol
30. 2,6-diisopropyl-phenol
31. Ici35,868
32. Mfcd00008885
33. Chembl526
34. Yi7vu623sf
35. Chebi:44915
36. Nsc5105
37. 2,6-di-iso-propylphenol-d18
38. Phenol, 2,6-bis(1-methylethyl)
39. Nsc-5105
40. Ici 35-868
41. Propofolum [latin]
42. Ncgc00015389-08
43. Aquafol
44. Cas-2078-54-8
45. Dsstox_cid_3523
46. Dsstox_rid_77063
47. Dsstox_gsid_23523
48. Diprivan Injectable Emulsion
49. 2,6 Diisopropylphenol
50. Dipravan
51. Propoven
52. Aquafo
53. Propofol Idd-d
54. Diprivan (tn)
55. Smr000059151
56. Ccris 9000
57. Propofol(2,6-diisopropylphenol)
58. Hsdb 7123
59. 2, 6-diisopropylphenol
60. Sr-01000075468
61. Einecs 218-206-6
62. 2,6-bis(isopropyl)phenol
63. Unii-yi7vu623sf
64. Brn 1866484
65. Dds-04f
66. Ghl.pd_mitscher_leg0.558
67. Ai3-26295
68. Propofol Solution
69. Propofol [usan:usp:inn:ban]
70. Propofol (diprivan)
71. Zd-0859
72. Phenol,6-diisopropyl-
73. Propofol [hsdb]
74. Propofol [usan]
75. Propofol [inn]
76. Propofol [jan]
77. Propofol [mi]
78. Propofol [vandf]
79. Prestwick0_000931
80. Prestwick1_000931
81. Prestwick2_000931
82. Prestwick3_000931
83. 2,6-di Isopropyl Phenol
84. Propofol [mart.]
85. 2,6-dipropan-2-ylphenol
86. Biomol-nt_000248
87. Lopac-d126608
88. Propofol [usp-rs]
89. Propofol [who-dd]
90. Diisopropylphenol (related)
91. Propofol (jan/usp/inn)
92. Lopac0_000437
93. Schembl36245
94. Bspbio_000862
95. 4-06-00-03435 (beilstein Handbook Reference)
96. Mls001066348
97. Mls001335999
98. Mls002454360
99. Bidd:gt0436
100. Propofol [green Book]
101. Spectrum1505022
102. Spbio_003031
103. 2,6-diisopropylphenol, 97%
104. Propofol [ep Impurity]
105. Propofol [orange Book]
106. Bpbio1_000950
107. Bpbio1_000969
108. Gtpl5464
109. Phenol,6-bis(1-methylethyl)-
110. Propofol [ep Monograph]
111. Propofol [usp Impurity]
112. Dtxsid6023523
113. Propofol [usp Monograph]
114. Ketafol Component Propofol
115. Propofol 1.0 Mg/ml In Methanol
116. 2,6-diisopropylphenol, >=97%
117. 3f33
118. 3p50
119. Hms1570l04
120. Hms2089o21
121. Hms2094e17
122. Hms2097l04
123. Hms2231e16
124. Hms3259e03
125. Hms3261g16
126. Hms3369i16
127. Hms3714l04
128. Pharmakon1600-01505022
129. Zinc968303
130. Albb-036351
131. Bcp02920
132. Hy-b0649
133. Tox21_110134
134. Tox21_201371
135. Tox21_303225
136. Tox21_500437
137. Ac8633
138. Bdbm50058046
139. Nsc758909
140. Phenol, 2, 6-bis(1-methylethyl)-
141. Akos009159417
142. Tox21_110134_1
143. Ac-2038
144. Am90311
145. Ccg-204529
146. Cs-w020057
147. Db00818
148. Lp00437
149. Nc00449
150. Nsc-758909
151. Sdccgmls-0318084.p029
152. Sdccgsbi-0050422.p002
153. 2,6-diisopropylphenol; Propofol
154. Mls-0318084
155. Ncgc00015389-01
156. Ncgc00015389-02
157. Ncgc00015389-03
158. Ncgc00015389-04
159. Ncgc00015389-05
160. Ncgc00015389-06
161. Ncgc00015389-07
162. Ncgc00015389-09
163. Ncgc00015389-10
164. Ncgc00015389-11
165. Ncgc00015389-14
166. Ncgc00015389-17
167. Ncgc00091538-01
168. Ncgc00091538-02
169. Ncgc00091538-03
170. Ncgc00091538-04
171. Ncgc00091538-05
172. Ncgc00091538-06
173. Ncgc00257228-01
174. Ncgc00260670-01
175. Ncgc00261122-01
176. As-13299
177. Sy013479
178. 2,6-diisopropylphenol, Analytical Standard
179. Bcp0726000298
180. Mls-0318084.p017
181. Ab00513968
182. D0617
183. Eu-0100437
184. C07523
185. D00549
186. Ab00513968-07
187. Ab00513968_08
188. A814898
189. D126608
190. Q422740
191. Q-201631
192. Sr-01000075468-1
193. Sr-01000075468-4
194. Sr-01000075468-6
195. Brd-k82255054-001-03-5
196. Brd-k82255054-001-08-4
197. Propofol, British Pharmacopoeia (bp) Reference Standard
198. Propofol, European Pharmacopoeia (ep) Reference Standard
199. Z1245735300
200. Propofol, United States Pharmacopeia (usp) Reference Standard
201. Propofol, Pharmaceutical Secondary Standard; Certified Reference Material
202. Propofol For Peak Identification, European Pharmacopoeia (ep) Reference Standard
203. Propofol Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
204. 113981-41-2
| Molecular Weight | 178.27 g/mol |
|---|---|
| Molecular Formula | C12H18O |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 178.135765193 g/mol |
| Monoisotopic Mass | 178.135765193 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 135 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Diprivan |
| PubMed Health | Propofol (Injection) |
| Drug Classes | Sedative-Hypnotic |
| Drug Label | For IV Administration OnlyRx OnlyStrict aseptic technique must always be maintained during handling. Diprivan Injectable Emulsion is a single-use parenteral product which contains 0.005% disodium edetate to inhibit the rate of growth of microorgani... |
| Active Ingredient | Propofol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Propofol |
| PubMed Health | Propofol (Injection) |
| Drug Classes | Sedative-Hypnotic |
| Drug Label | Propofol injectable emulsion is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6-diisopropylphenol and has a molecular weight of 178.27. The structural a... |
| Active Ingredient | Propofol |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 10mg/ml |
| Market Status | Tentative Approval; Prescription |
| Company | Hospira; Teva Pharms Usa; Gensia Sicor Pharms; Baxter Hlthcare; Esi Lederle |
| 3 of 4 | |
|---|---|
| Drug Name | Diprivan |
| PubMed Health | Propofol (Injection) |
| Drug Classes | Sedative-Hypnotic |
| Drug Label | For IV Administration OnlyRx OnlyStrict aseptic technique must always be maintained during handling. Diprivan Injectable Emulsion is a single-use parenteral product which contains 0.005% disodium edetate to inhibit the rate of growth of microorgani... |
| Active Ingredient | Propofol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Propofol |
| PubMed Health | Propofol (Injection) |
| Drug Classes | Sedative-Hypnotic |
| Drug Label | Propofol injectable emulsion is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6-diisopropylphenol and has a molecular weight of 178.27. The structural a... |
| Active Ingredient | Propofol |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 10mg/ml |
| Market Status | Tentative Approval; Prescription |
| Company | Hospira; Teva Pharms Usa; Gensia Sicor Pharms; Baxter Hlthcare; Esi Lederle |
Propofol is indicated for the induction of general anesthesia. It is also indicated for the maintenance of anesthesia utilizing balanced techniques with other appropriate agents such as opioids and inhalation anesthetics.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2466
Propofol is indicated for the sedation in critically ill patients confined to intensive care units.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2466
Propofol has successfully controlled seizures in status epilepticus.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1181
Propofol appears to have significant antiemetic action and is a good choice for sedation or anesthesia in patients at high risk for nausea and vomiting.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 345
Compromised cardiovascular function may be aggravated by cardiovascular-depressant and hypotensive effects.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
Caution is also recommended in geriatric, debilitated, and/or hypovolemic patients, because they may require lower induction and maintenance doses.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2468
Substantial decreases in mean arterial pressure and cerebral perfusion may occur in patients with existing impaired cerebral circulation or increased intracranial pressure.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2468
Rarely, a clinical syndrome including bronchospasm, erythema, and hypotension has occured shortly after administration of propofol, and sequelae including anoxic brain damage and death have been reported. /SRP: May be from metabisulfite./
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2468
For more Drug Warnings (Complete) data for PROPOFOL (17 total), please visit the HSDB record page.
Used for induction and/or maintenance of anaesthesia and for management of refractory status epilepticus.
FDA Label
Propofol is a sedative-hypnotic agent for use in the induction and maintenance of anesthesia or sedation. Intravenous injection of a therapeutic dose of propofol produces hypnosis rapidly with minimal excitation, usually within 40 seconds from the start of an injection (the time for one arm-brain circulation).
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N01AX10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AX - Other general anesthetics
N01AX10 - Propofol
Absorption
Rapid - time to onset of unconsciousness is 15-30 seconds, due to rapid distribution from plasma to the CNS. Distribution is so rapid that peak plasma concentrations cannot be readily measured. Duration of action is 5-10 minutes.
Route of Elimination
It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney.
Volume of Distribution
60 L/kg [healthy adults]
Clearance
23 - 50 mL/kg/min
1.6 - 3.4 L/min [70 Kg adults]
The initial apparent volume of distribution is 13 to 76 L/kg.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
Propofol is rapidly and extensively distributed in the body. It crosses the blood-brain barrier quickly, and its short duration of action is due to rapid redistribution from the CNS to other tissues, high metabolic clearance and high lipophilicity.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
Approximately 70% of a dose is excreted in the urine within 24 hours after administration, and 90% is excreted within 5 days. Clearance of propofol ranges from 1.6 to 3.4 liters per minute in healthy 70 kg patients. As the age of the patient increases, total clearance of propofol may decrease. Clearance rates of 1.4 to 2.2 liters per minute in patients 18 to 35 years of age have been reported, in contrast to clearance rates of 1.0 to 1.8 liters per minute in patients 65 to 80 years of age.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
The pharmacokinetics of propofol were best described by a three-compartment model. Weight was found to be a significant covariate for elimination clearance, the two intercompartmental clearances, and the volumes of the central compartment, the shallow peripheral compartment, and the deep peripheral compartment; power functions with exponents smaller than 1 yielded the best results. The estimates of these parameters for a 70-kg adult were 1.44 l/min, 2.25 l/min, 0.92 l/min, 9.3 l, 44.2 l, and 266 l, respectively. For patients older than 60 yr the elimination clearance decreased linearly. The volume of the central compartment decreased with age. For children, all parameters were increased when normalized to body weight. Venous data showed a decreased elimination clearance; bolus data were characterized by increases in the volumes of the central and shallow peripheral compartments and in the rapid distribution clearance (Cl2) and a decrease in the slow distribution clearance (Cl3). Pharmacokinetics of propofol can be well described by a three-compartment model. Inclusion of age and weight as covariates significantly improved the model. Adjusting pharmacokinetics to the individual patient should improve the precision of target-controlled infusion and may help to broaden the field of application for target-controlled infusion systems.
PMID:10719952 Schuttler J, Ihmsen H; Anesthesiology 92 (3): 727-38 (2000)
An iv dose of 14C-propofol (0.47 mg/kg) administered to 6 male volunteers was rapidly eliminated with 88% recovered in the urine in 5 days and <2% in feces. The dose was cleared by metabolism with <0.3% excreted unchanged. The major metabolites were the glucuronic acid conjugate of propofol and the glucuronic acid and sulfate conjugates of its hydroxylated derivative, 2,6-diisopropyl-1,4-quinol. Propofol glucuronide accounted for about 53% of the urinary radioactivity and was the major metabolite in plasma from 30 min post dose. The blood concentration of propofol declined in a biphasic manner from a maximum mean value of 0.44 ug/ml, 2 min after injection. The half-lives of the first and second exponential phases, mean values 5 min and 97 min respectively, varied widely among subjects. A proportion of the dose was cleared slowly, probably due to slow release from less well perfused tissues. Propofol accounted for 94% of the total blood radioactivity at 2 min but only about 6% from 3 to 8 hr post dose. Propofol has a volume of distribution equivalent to about 3 to 4 times body weight, and a mean total body clearance of 2.2 l/min.
PMID:3261062 Simons P, Cockshott I; Xenobiotica 18 (4): 429-40 (1988)
Hepatically metabolized mainly by glucuronidation at the C1-hydroxyl. Hydroxylation of the benzene ring to 4-hydroxypropofol may also occur via CYP2B6 and 2C9 with subsequent conjugation to sulfuric and/or glucuronic acid. Hydroxypropofol has approximately 1/3 of hypnotic activity of propofol.
Hepatic; rapidly undergoes glucuronide conjugation to inactive metabolites. An unidentified route of extrahepatic metabolism may also exist, suggested by the fact that propofol clearance exceeds estimated hepatic blood flow.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
To determine the cytochrome P450 (CYP) isoforms involved in the oxidation of propofol by human liver microsomes. The rate constant calculated from the disappearance of propofol in an incubation mixture with human liver microsomes and recombinant human CYP isoforms was used as a measure of the rate of metabolism of propofol. The correlation of these rate constants with rates of metabolism of CYP isoform-selective substrates by liver microsomes, the effect of CYP isoform-selective chemical inhibitors and monoclonal antibodies on propofol metabolism by liver microsomes, and its metabolism by recombinant human CYP isoforms were examined. The mean rate constant of propofol metabolism by liver microsomes obtained from 6 individuals was 4.2 (95% confidence intervals 2.7, 5.7) nmol/min/mg protein. The rate constants of propofol by microsomes were significantly correlated with S-mephenytoin N-demethylation, a marker of CYP2B6 (r=0.93, P<0.0001), but not with the metabolic activities of other CYP isoform-selective substrates. Of the chemical inhibitors of CYP isoforms tested, orphenadrine, a CYP2B6 inhibitor, reduced the rate constant of propofol by liver microsomes by 38% (P<0.05), while other CYP isoform-selective inhibitors had no effects. Of the recombinant CYP isoforms screened, CYP2B6 produced the highest rate constant for propofol metabolism (197 nmol/min/nmol P450). An antibody against CYP2B6 inhibited the disappearance of propofol in liver microsomes by 74% /and SRP: reduced in vitro metabolism by blocking CYP 2B6/. Antibodies raised against other CYP isoforms had no effect on the metabolism of propofol. CYP2B6 is predominantly involved in the oxidation of propofol by human liver microsomes.
Oda Y, Hamaoka N; Br J Clin Pharmacol 51 (3): 281-5 (2001
Propofol has known human metabolites that include (2S,3S,4S,5R)-6-[2,6-Di(propan-2-yl)phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid and 4-hydroxy-propofol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Initial distribution phase t1/2α=1.8-9.5 minutes. Second redistirubtion phase t1/2β=21-70 minutes. Terminal elimination phase t1/2γ=1.5-31 hours.
Terminal elimination half-life is 3 to 12 hours; prolonged administration may result in longer duration.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2467
...The first-stage elimination half-life (t1/2 beta) of propofol /SRP: administered mixed with lidocaine/ in children was shorter (mean 9.3 +/- 3.8 (s.d.) min) than the values found in adults. This pharmacokinetic alteration may have clinical significance following repeated administration or continuous infusion of propofol.
PMID:2646847 Valtonen M, et al; Acta Anaesthesiol Scand 33 (2): 152-5 (1989)
An intravenous dose of 14C-propofol (0.47 mg/kg) /was/ administered to six male volunteers... . ...The half-lives of the first and second exponential phases, mean values 5 min and 97 min respectively, varied widely among subjects.
PMID:3261062 Simons P, Cockshott I; Xenobiotica 18 (4): 429-40 (1988)
The action of propofol involves a positive modulation of the inhibitory function of the neurotransmitter gama-aminobutyric acid (GABA) through GABA-A receptors.


