



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
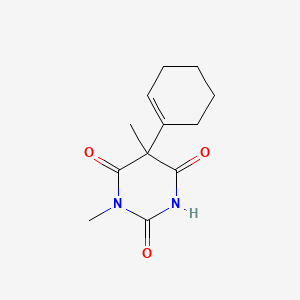

1. Evipan
2. Hexenal
3. Hexobarbital, Sodium
4. Hexobarbitone
5. Sodium Hexobarbital
1. Hexobarbitone
2. Methylhexabital
3. Methexenyl
4. 56-29-1
5. Evipal
6. Evipan
7. Barbidorm
8. Cyclopan
9. Enhexymal
10. Noctivane
11. Sombucaps
12. Sombulex
13. Somnalert
14. Citopan
15. Methylhexabarbital
16. Cyclonal
17. Hexabarbital
18. Narcosan
19. Citodon
20. Dorico
21. Hexanastab Oral
22. Hexenal (barbiturate)
23. Esobarbitale
24. Hexobarbitalum
25. 5-(1-cyclohexen-1-yl)-1,5-dimethylbarbituric Acid
26. N-methyl-5-cyclohexenyl-5-methylbarbituric Acid
27. 1,5-dimethyl-5-(1-cyclohexenyl)barbituric Acid
28. Hexobarbital Ciii
29. 5-(cyclohexen-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione
30. Nsc 71929
31. 5-(1-cyclohexen-1-yl)-1,5-dimethyl-2,4,6(1h,3h,5h)-pyrimidinetrione
32. 5-(1-cyclohexenyl-1)-1-methyl-5-methylbarbituric Acid
33. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-cyclohexen-1-yl)-1,5-dimethyl-
34. Nsc-71929
35. Al8z8k3p6s
36. Barbituric Acid, 5-(1-cyclohexen-1-yl)-1,5-dimethyl-
37. Chebi:5706
38. Enhexymalum
39. Hexobarbitonum
40. 5-(cyclohex-1-en-1-yl)-1,5-dimethylpyrimidine-2,4,6(1h,3h,5h)-trione
41. Ncgc00159430-02
42. Ncgc00159430-03
43. Ncgc00159430-04
44. Hexobarbital (van)
45. Esobarbitale [dcit]
46. Esobarbitale [italian]
47. Dsstox_cid_3122
48. Dsstox_rid_76882
49. Dsstox_gsid_23122
50. Hexobarbitalum [inn-latin]
51. 5-cyclohex-1-enyl-1,5-dimethyl-pyrimidine-2,4,6-trione
52. 5-(.delta.-1,2-cyclohexenyl)-5-methyl-n-methyl-barbitursaeure
53. 5-cyclohexenyl-1,5-dimethylpyrimidine-2,4,6(1h,3h,5h)-trione
54. 5-(cyclohex-1-en-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione
55. Cas-56-29-1
56. Einecs 200-264-9
57. Hexobarbital (jan/inn)
58. Unii-al8z8k3p6s
59. Ai3-61892
60. Hexobarbital [usp:inn:ban:jan]
61. 5-(delta-1,2-cyclohexenyl)-5-methyl-n-methyl-barbitursaeure [german]
62. (+/-)-hexobarbital
63. 5-(delta-1,2-cyclohexenyl)-5-methyl-n-methyl-barbitursaeure
64. Hexobarbital [mi]
65. Hexobarbital [inn]
66. Hexobarbital [jan]
67. Barbituric Acid,5-dimethyl-
68. Chembl7728
69. Hexobarbital [vandf]
70. Oprea1_350917
71. Schembl29821
72. Hexobarbital [mart.]
73. Mls001143909
74. Hexobarbital [who-dd]
75. Dtxsid9023122
76. Hexobarbital [ep Impurity]
77. Hms2272k03
78. Nsc71929
79. Hexobarbital 1.0 Mg/ml In Methanol
80. Tox21_111661
81. Tox21_111662
82. Stk696034
83. 5-cyclohex-1-en-1-yl-1,5-dimethylpyrimidine-2,4,6(1h,3h,5h)-trione
84. 5-cyclohex-1-en-1-yl-2-hydroxy-1,5-dimethylpyrimidine-4,6(1h,5h)-dione
85. Akos000355976
86. Akos016347605
87. Tox21_111661_1
88. Db01355
89. Ac-16076
90. Smr000473348
91. Db-052887
92. Wln: T6vmvnv Fhj D1 F1 F- Al6utj
93. D01071
94. 5-(cyclohexen-1-yl)-1,5-dimethylbarbitursaeure
95. 5-(cyclohexen-1-yl)-1,5-dimethylbarbituric Acid
96. Q421183
97. 5-(cyclohex-1-enyl)-1,5-dimethyl-barbituric Acid
98. Hexobarbital, United States Pharmacopeia (usp) Reference Standard
99. 2,6(1h,3h,5h)-pyrimidinetrione, 5-(1-cyclohexen-1-yl)-1,5-dimethyl-
100. 5-(1-cyclohexenyl)-1,5-dimethyl-2,4,6(1h,3h,5h)-pyrimidinetrione
101. Hexobarbital Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 236.27 g/mol |
|---|---|
| Molecular Formula | C12H16N2O3 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 236.11609238 g/mol |
| Monoisotopic Mass | 236.11609238 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the induction of anesthesia prior to the use of other general anesthetic agents and for induction of anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli.
Hexobarbital is a barbiturate derivative having hypnotic and sedative effects. It was subsequently used in the 1940s and 1950s as an anesthetic for surgery. Furthermore, the agent also demonstrates a fairly quick onset of action that also possesses a short duration of action. However it can be difficult to control the depth of anesthesia with hexobarbital which makes it quite dangerous, and it has now been replaced by safer drugs in human medicine, usually thiopental would be the barbiturate of choice for this application these days.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AF - Barbiturates, plain
N01AF02 - Hexobarbital
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA16 - Hexobarbital
Hepatic.
Hexobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABA-A receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.


