



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
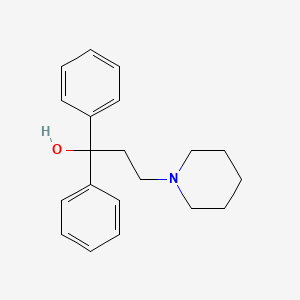

1. 3-(n-piperidinyl)-1,1-diphenyl-1-propanol Methanesulfonate
2. 3-piperidino-1,1-diphenylpropanol
3. 3-piperidinyl-1,1-diphenylpropan-1-ol
4. Myoson
5. Pridinol Mesylate
6. Pridinol Monohydrochloride
7. Pridinol Monomesylate
8. Ridinol
1. 511-45-5
2. 1,1-diphenyl-3-(piperidin-1-yl)propan-1-ol
3. Nonplesin
4. Lyseen
5. Parks
6. Parks 12
7. Parks 12 Hommel
8. Pridinolum
9. Nonpressin (free Base)
10. C-238
11. Hh 212
12. 1,1-diphenyl-3-piperidino-1-propanol
13. Alpha,alpha-diphenyl-1-piperidinepropanol
14. 1,1-diphenyl-3-(1-piperidyl)-1-propanol
15. 1-piperidinepropanol, Alpha,alpha-diphenyl-
16. 3-(n-piperidyl)-1,1-diphenyl-1-propanol
17. 238 C
18. Nsc 23016
19. Nsc-23016
20. Nsc23016
21. 9e75q6suub
22. 1,1-diphenyl-3-piperidin-1-ylpropan-1-ol
23. 1,1-diphenyl-3-(1-piperidinyl)-1-propanol
24. Ridinol
25. Chebi:75247
26. Pridinol (inn)
27. Pridinol Mesilate
28. Benzhydrol, .alpha.-(2-piperidinoethyl)-
29. Pridinol [inn]
30. Nsc 23016; Nsc 403797; Nonplesin
31. .alpha.,.alpha.-diphenyl-1-piperidinepropanol
32. Dsstox_cid_25090
33. Dsstox_rid_80664
34. Dsstox_gsid_45090
35. Pridinol [inn:dcf]
36. Pridinolum [inn-latin]
37. 1-piperidinepropanol,.alpha.-diphenyl-
38. Cas-511-45-5
39. Hsdb 2684
40. Einecs 208-128-0
41. Unii-9e75q6suub
42. Benzhydrol, Alpha-(2-piperidinoethyl)-
43. Brn 0252983
44. A-carotene
45. Spectrum_001320
46. Pridinol [hsdb]
47. Pridinol [mi]
48. Prestwick0_000799
49. Prestwick1_000799
50. Prestwick2_000799
51. Prestwick3_000799
52. Spectrum2_001985
53. Spectrum3_001552
54. Spectrum4_000728
55. Spectrum5_001069
56. Pridinol [who-dd]
57. Ncistruc1_000328
58. Ncistruc2_000375
59. Bspbio_000938
60. Bspbio_003024
61. Kbiogr_000996
62. Kbioss_001800
63. 5-20-02-00247 (beilstein Handbook Reference)
64. Divk1c_000726
65. Schembl143298
66. Spbio_002169
67. Spbio_002877
68. Bpbio1_001032
69. Chembl404215
70. Dtxsid0045090
71. Kbio1_000726
72. Kbio2_001800
73. Kbio2_004368
74. Kbio2_006936
75. Kbio3_002524
76. Ninds_000726
77. Alpha-(2-piperidinoethyl)benzhydrol
78. Nci23016
79. Zinc1482149
80. Tox21_110022
81. Ccg-37974
82. Mfcd00242961
83. Ncgc00013289
84. Nsc403797
85. Akos016001547
86. Tox21_110022_1
87. Db13642
88. Nsc-403797
89. Idi1_000726
90. Ncgc00013289-01
91. Ncgc00013289-02
92. Ncgc00013289-03
93. Ncgc00013289-04
94. Ncgc00013289-05
95. Ncgc00013289-06
96. Ncgc00013289-07
97. Ncgc00013289-09
98. Ncgc00023906-03
99. Ncgc00023906-04
100. Ncgc00023906-05
101. Ac-24366
102. Nci60_001871
103. Sbi-0051765.p002
104. Ab00053638
105. 1,1-diphenyl-3-piperidin-1-yl-propan-1-ol
106. D08418
107. 1,1-diphenyl-3-(1-piperidinyl)-1-propanol #
108. 1-piperidinepropanol, .alpha.,.alpha.-diphenyl-
109. Ab00053638_16
110. Ab00053638_17
111. N-propanol, 1,1-diphenyl-3-(piperidin-1-yl)-
112. Ag-680/20240026
113. Q827515
114. Brd-k17565903-066-05-8
115. Brd-k17565903-066-15-7
| Molecular Weight | 295.4 g/mol |
|---|---|
| Molecular Formula | C20H25NO |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 295.193614421 g/mol |
| Monoisotopic Mass | 295.193614421 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 294 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ A randomized, double-blind, placebo-controlled three-way cross-over study was performed to investigate the effect of two muscle relaxants (tolperisone hydrochloride and pridinol mesilate) on experimental jaw-muscle pain and jaw-stretch reflexes. Fifteen healthy men participated in three randomized sessions separated by at least 1 week. In each session 300 mg tolperisone, 8 mg pridinol mesilate or placebo was administered orally as a single dose. One hour after drug administration 0.3 mL hypertonic saline (5.8%) was injected into the right masseter to produce muscle pain. Subjects continuously rated their perceived pain intensity on an electronic 10-cm visual analogue scale (VAS). The pressure pain threshold (PPT) was measured and short-latency reflex responses were evoked in the pre-contracted (15% maximal voluntary contraction) masseter and temporalis muscles by a standardised stretch device (1 mm displacement, 10 ms ramp time) before (baseline), 1 hr after medication (post-drug), during ongoing experimental muscle pain (pain-post-drug), and 15 min after pain had vanished (post-pain). Analysis of variance demonstrated significantly lower VAS peak pain scores (5.9 +/- 0.4 cm) after administration of tolperisone hydrochloride compared with pridinol mesilate (6.8 +/- 0.4 cm) and placebo (6.6 +/- 0.4 cm) (P=0.020). Administration of pridinol mesilate was associated with a significant decrease in PPTs compared with tolperisone hydrochloride and placebo (P=0.002) after medication, but not after experimental jaw-muscle pain. The normalized peak-to-peak amplitude of the stretch reflexes were not significantly influenced by the test medication (P=0.762), but were in all sessions significantly facilitated during ongoing experimental jaw-muscle pain (P=0.034). In conclusion, tolperisone hydrochloride provides a small, albeit significant reduction in the perceived intensity of experimental jaw-muscle pain whereas the present dose had no effect on the short-latency jaw-stretch reflex. /Tolperisone hydrochloride and pridinol mesilate/
PMID:12935797 Svensson P et al; Eur J Pain 7 (5): 449-56 (2003)
M - Musculo-skeletal system
M03 - Muscle relaxants
M03B - Muscle relaxants, centrally acting agents
M03BX - Other centrally acting agents
M03BX03 - Pridinol


