



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
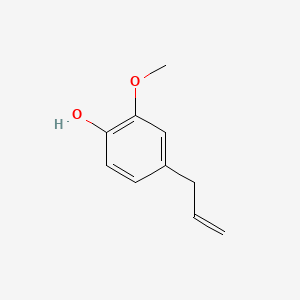

1. Phenol, 2-methoxy-4-(2-propenyl)-
1. 97-53-0
2. 4-allyl-2-methoxyphenol
3. 4-allylguaiacol
4. Eugenic Acid
5. Allylguaiacol
6. Caryophyllic Acid
7. P-allylguaiacol
8. 2-methoxy-4-prop-2-enylphenol
9. P-eugenol
10. Engenol
11. 2-methoxy-4-allylphenol
12. Phenol, 2-methoxy-4-(2-propenyl)-
13. 2-methoxy-4-(2-propenyl)phenol
14. 1,3,4-eugenol
15. 4-allylcatechol-2-methyl Ether
16. 1-hydroxy-2-methoxy-4-allylbenzene
17. 5-allylguaiacol
18. Synthetic Eugenol
19. 2-methoxy-1-hydroxy-4-allylbenzene
20. 4-allyl-1-hydroxy-2-methoxybenzene
21. 1-hydroxy-2-methoxy-4-prop-2-enylbenzene
22. 2-methoxy-4-(prop-2-en-1-yl)phenol
23. Eugenol (natural)
24. Fema No. 2467
25. 4-hydroxy-3-methoxy-1-allylbenzene
26. 2-hydroxy-5-allylanisole
27. Bioxeda
28. Dentogum
29. 4-allylcatechol 2-methyl Ether
30. 4-hydroxy-3-methoxyallylbenzene
31. 2-methoxy-4-(2-propen-1-yl)phenol
32. Phenol, 4-allyl-2-methoxy-
33. Nci-c50453
34. 1-allyl-4-hydroxy-3-methoxybenzene
35. 1-allyl-3-methoxy-4-hydroxybenzene
36. 2-metoksy-4-allilofenol
37. Caryophillic Acid
38. Fa 100
39. Eugenol [usp]
40. Nsc 209525
41. Chebi:4917
42. Phenol, 2-methoxy-4-(2-propen-1-yl)-
43. Nsc-8895
44. Nsc-209525
45. Chembl42710
46. 2-methoxy-4-(3-propenyl)phenol
47. 3t8h1794qw
48. Eugenol (usp)
49. Ncgc00091449-05
50. Dsstox_cid_617
51. Dsstox_rid_75693
52. Dsstox_gsid_20617
53. Eugenol [usan]
54. Wln: 1u2r Dq Co1
55. Phenol, 2-methoxy-4-(2-propen-1-yl)-, Homopolymer
56. Caswell No. 456bc
57. Fema Number 2467
58. Cas-97-53-0
59. 38219-15-7
60. Ccris 306
61. Hsdb 210
62. 2-metoksy-4-allilofenol [polish]
63. Sr-05000002043
64. Einecs 202-589-1
65. Mfcd00008654
66. Epa Pesticide Chemical Code 102701
67. Brn 1366759
68. Unii-3t8h1794qw
69. Ai3-00086
70. Eugenol,(s)
71. 4-allyl-2methoxyphenol
72. 3s0e
73. Eugenol [vandf]
74. Eugenol [fhfi]
75. Eugenol [hsdb]
76. Eugenol [iarc]
77. Eugenol [inci]
78. Eugenol [fcc]
79. Eugenol [ii]
80. Eugenol [mi]
81. Eugenol [mart.]
82. Spectrum2_001264
83. Spectrum3_000646
84. Spectrum4_001783
85. Spectrum5_000425
86. Eugenol [usp-rs]
87. Eugenol [who-dd]
88. 4-allyl-2-methoxy-phenol
89. Bmse010053
90. Epitope Id:114091
91. Eugenol, Puriss., 98%
92. Ec 202-589-1
93. Schembl20361
94. Bspbio_002251
95. Kbiogr_002327
96. Mls000028901
97. Bidd:er0696
98. Divk1c_000692
99. Spectrum1500296
100. Spbio_001228
101. Eugenol [ep Monograph]
102. Gtpl2425
103. Zinc1411
104. Eugenol [usp Monograph]
105. Dtxsid9020617
106. Hms502c14
107. Kbio1_000692
108. Kbio3_001471
109. Eugenol, Reagentplus(r), 99%
110. Nsc8895
111. 4-(2-propenyl)-2-methoxyphenol
112. Eugenol, Natural, >=98%, Fg
113. Ninds_000692
114. Eugenol, >=98%, Fcc, Fg
115. Hms1920o08
116. Hms2091f09
117. Pharmakon1600-01500296
118. Hy-n0337
119. Tox21_111134
120. Tox21_202040
121. Tox21_300105
122. Bbl027721
123. Bdbm50164168
124. Ccg-38827
125. Nsc209525
126. Nsc757030
127. S4706
128. Stl371304
129. Eugenol, Tested According To Ph.eur.
130. 3-(3-methoxy-4-hydroxyphenyl)propene
131. Akos000121354
132. Tox21_111134_1
133. Cs-7807
134. Db09086
135. Fs-2702
136. Nsc-757030
137. Sdccgmls-0066578.p001
138. Idi1_000692
139. Eugenol 1000 Microg/ml In Acetonitrile
140. Ncgc00091449-01
141. Ncgc00091449-02
142. Ncgc00091449-03
143. Ncgc00091449-04
144. Ncgc00091449-06
145. Ncgc00091449-07
146. Ncgc00091449-08
147. Ncgc00091449-10
148. Ncgc00253915-01
149. Ncgc00259589-01
150. Ac-34149
151. Eugenol, Vetec(tm) Reagent Grade, 98%
152. Smr000059114
153. Sbi-0051381.p003
154. Eugenol, Pestanal(r), Analytical Standard
155. A0232
156. Ft-0615974
157. N1805
158. D04117
159. Ab00051992_02
160. A845719
161. Eugenol, Primary Pharmaceutical Reference Standard
162. Q423357
163. Eugenol, Certified Reference Material, Tracecert(r)
164. Q-201105
165. Sr-05000002043-1
166. Sr-05000002043-2
167. Brd-k32977963-001-01-9
168. Brd-k32977963-001-03-5
169. Eugenol (constituent Of Holy Basil Leaf) [dsc]
170. Eugenol, European Pharmacopoeia (ep) Reference Standard
171. F0001-2306
172. 2-methoxy-4-(prop-2-en-1-yl)phenol4-allyl-2-methoxyphenol
173. Eugenol (constituent Of Cinnamomum Cassia Bark) [dsc]
174. Eugenol (constituent Of Cinnamomum Verum Bark) [dsc]
175. Eugenol, United States Pharmacopeia (usp) Reference Standard
176. Eugenol, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 164.20 g/mol |
|---|---|
| Molecular Formula | C10H12O2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 164.083729621 g/mol |
| Monoisotopic Mass | 164.083729621 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 145 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... Has been used as an antipyretic but it is relatively ineffective. /Eugenol/ has... been used in medicine for the study of mucous secretion /and/ gastric cytology, without gastric resection or gastroenterostomy. It has been shown to have anthelmintic properties. /SRP: Former use/
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1691
Nonprescription medicines for toothache commonly contain eugenol, and some products for canker-sore may do so also.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V36 78 (1985)
Eugenol is used as a component of several dental materials (e.g., dental cements, impression pastes and surgical pastes). Such products are principally combinations of zinc oxide and eugenol in varying ratios. They are reported to be widely used in dentistry as temporary filing materials, cavity liners for pulp protection, capping materials, temporary cementation of fixed protheses, impression materials and major ingredients of endodontic sealers. In addition, eugenol has been used in dentistry for disinfecting root canals.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V36 77 (1985)
Analgesic (dental)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 690
Eugenol is not currently available in any FDA-approved drug products. There are a number of unapproved OTC products that advertise it for the use of toothache. Eugenol is is also commonly used in combination with zinc oxide in dental procedures for the cementation of temporary prostheses and the temporary restoration of teeth and cavities.
FDA Label
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Solvents
Liquids that dissolve other substances (solutes), generally solids, without any change in chemical composition, as, water containing sugar. (Grant and Hackh's Chemical Dictionary, 5th ed) (See all compounds classified as Solvents.)
Intraperitoneal injection of a single 450 mg/kg dose of (14)C methoxy labelled eugenol resulted in rapid distribution to all organs. Both ether- and water soluble materials were recovered from most tissues and excretions. Only 0.2-1.0% of the dose was eliminated as expired (14)CO2. Over 70% of a lethal dose of eugenol was recovered on death, from the urine of rabbits.
WHO; Food Additive Series 17: Eugenol (1980). Available from, as of April 21, 2005: https://www.inchem.org/documents/jecfa/jecmono/v17je10.htm
No penetration of mouse skin was demonstrated after dermal application of eugenol.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V36 86 (1985)
Following ip injection of (14)C eugenol into rats, ... the presence of (14)CO2 in expired air indicated the demethylation of eugenol.
Opdyke, D.L.J. (ed.). Monographs on Fragrance Raw Materials. New York: Pergamon Press, 1979., p. 376
The metabolism and toxic effects of eugenol were studied in isolated rat hepatocytes. Incubation of hepatocytes with eugenol resulted in the formation of conjugates with sulfate, glucuronic acid and glutathione. The major metabolite formed was the glucuronic acid conjugate. Covalent binding to cellular protein was observed using (3)H eugenol. Loss of intracellular glutathione and cell death were also observed in these incubations. Concentrations of 1 mM eugenol caused a loss of over 90% of intracellular glutathione and resulted in approximately 85% cell death over a 5 hr incubation period. The loss of the majority of glutathione occurred prior to the onset of cell death (2 hr). The effects of eugenol were concentration dependent. The addition of 1 mM N-acetylcysteine to incubations containing 1 mM eugenol was able to completely prevent glutathione loss and cell death as well as inhibit the covalent binding of eugenol metabolites to protein. Conversely, pretreatment of hepatocytes with diethylmaleate to deplete intracellular glutathione increased the cytotoxic effects of eugenol. These results demonstrate that eugenol is actively metabolized in hepatocytes and suggest that the cytotoxic effects of eugenol are due to the formation of a reactive intermediate, possibly a quinone methide.
PMID:1991333 Thompson DC et al; Chem Biol Interact 77 (2): 137-47 (1991)
Two metabolites of eugenol, 3-piperidyl-1-(3'-methoxy-4'-hydroxyphenyl)-1-propanone and 3-pyrrolidinyl-1-(3'-methoxy-4'-hydroxyphenyl)-1-propanone, have been isolated from rat urine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V36 86 (1985)
Epoxidation of eugenol by rat liver cell cultures has been reported. The dihydrodiol metabolite of eugenol has been isolated from liver homogenates and urine of rats pretreated with eugenol.
WHO; Food Additive Series 17: Eugenol (1980). Available from, as of April 21, 2005: https://www.inchem.org/documents/jecfa/jecmono/v17je10.htm
For more Metabolism/Metabolites (Complete) data for EUGENOL (9 total), please visit the HSDB record page.
Eugenol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-(2-methoxy-4-prop-2-enylphenoxy)oxane-2-carboxylic acid and Hydroxychavicol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The exact mechanism of action of eugenol is unknown. However, eugenol has been shown to interrupt action potentials, which may be involved in its anti-pain activity. Research has also shown eugenol to have anti-inflammatory, neuroprotective, antipyretic, antioxidant, antifungal and analgesic properties.
... Thymocyte suspension was irradiated by gamma-rays, and the malondialdehyde (MDA) formation was measured with the thiobarbituric acid reactive species (TBARS) method. The results showed an increase in MDA in irradiated (2 Gy) thymocytes, which was inhibited in samples treated with increasing concentrations of eugenol (10-200 uM) prior to irradiation. The concentration of eugenol required to inhibit half of the MDA formation (IC(50)) in irradiated thymocytes was 100 uM. A dose-dependent increase in the generation of ROS was observed in irradiated thymocytes (0.5-200 cGy) as measured by 2,7-dichlorodihydro fluorescein diacetate (DCH-FDA), which was inhibited by eugenol administered before irradiation.
PMID:15163290 Pandy BN, Mishra KP; J Environ Pathol Toxicol Oncol 23 (2): 117-22 (2004)
Respiratory inhibition of isolated rat liver mitochondria by eugenol was dose related and uncoupled oxidative phosphorylation from electron transfer.
PMID:295190 Cotmore JM et al; Arch Oral Biol 24 (8): 565 (1979)
Polymorphonuclear leukocytes (PMNL) play an important role in the modulation of inflammatory conditions in humans. PMNL cells recruited at the site of inflammation, release inflammatory mediators such as leukotrienes, proteolytic enzymes and reactive oxygen species. Among these, leukotrienes are implicated in pathophysiology of allergic and inflammatory disorders like asthma, allergic rhinitis, arthritis, inflammatory bowel disease and psoriasis. 5-lipoxygenase (5-LO) is the key enzyme in biosynthetic pathway of leukotrienes. Our earlier studies showed that spice phenolic active principles significantly inhibit 5-LO enzyme in human PMNLs. In this study we have further characterized the inhibitory mechanism of eugenol, the active principle of spice-clove on 5-LO enzyme and also its effect on leukotriene C((4)) (LTC(4)). Substrate dependent enzyme kinetics showed that the inhibitory effect of eugenol on 5-LO was of a non-competitive nature. Further, eugenol was found to significantly inhibit the formation of LTC(4) in calcium ionophore A23187 and arachidonic acid (AA) stimulated PMNL cells. These data clearly suggest that eugenol inhibits 5-LO by non-competitive mechanism and also inhibits formation of LTC(4) in human PMNL cells and thus may have beneficial role in modulating 5-LO pathway in human PMNL cells.
PMID:16216483 Raghavenra H et al; Prostaglandins Leukot Essent Fatty Acids 74 (1): 23-7 (2006)
The Ca(2+)-activated Cl(-) channel TMEM16A is involved in epithelial fluid secretion, smooth muscle contraction and neurosensory signaling. We identified a Thai herbal antidiarrheal formulation that inhibited TMEM16A Cl(-) conductance. C18-reversed-phase HPLC fractionation of the herbal formulation revealed >98% of TMEM16A inhibition activity in one out of approximately 20 distinct peaks. The purified, active compound was identified as eugenol (4-allyl-2-methoxyphenol), the major component of clove oil. Eugenol fully inhibited TMEM16A Cl(-) conductance with single-site IC(50)~150 uM. Eugenol inhibition of TMEM16A in interstitial cells of Cajal produced strong inhibition of intestinal contraction in mouse ileal segments. TMEM16A Cl(-) channel inhibition adds to the list of eugenol molecular targets and may account for some of its biological activities.
PMID:22666439 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3364195 Yao Z et al; PLoS One. 2012;7(5):e38030. doi: 10.1371/journal.pone.0038030. Epub 2012 May 30.
For more Mechanism of Action (Complete) data for EUGENOL (21 total), please visit the HSDB record page.


