



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
JP
0
Other Listed Suppliers
0
0
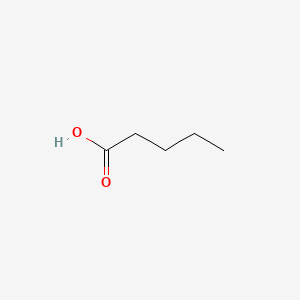

1. Lithium Pentanoate
2. N-pentanoic Acid
3. N-pentanoic Acid, 11c-labeled
4. N-pentanoic Acid, 11c-labeled Sodium Salt
5. N-pentanoic Acid, Ammonium Salt
6. N-pentanoic Acid, Maganese (+2) Salt
7. N-pentanoic Acid, Potassium Salt
8. N-pentanoic Acid, Sodium Salt
9. N-pentanoic Acid, Zinc Salt
1. Pentanoic Acid
2. N-valeric Acid
3. 109-52-4
4. N-pentanoic Acid
5. Valerianic Acid
6. 1-butanecarboxylic Acid
7. Propylacetic Acid
8. Butanecarboxylic Acid
9. Pentoic Acid
10. Kyselina Valerova
11. Valeric Acid, N-
12. Valeric Acid, Normal
13. N-pentanoate
14. Kyselina Valerova [czech]
15. Valerate
16. Fema No. 3101
17. Valeriansaeure
18. N-valerate
19. 1-pentanoic Acid
20. Nsc 406833
21. N-c4h9cooh
22. Gzk92pjm7b
23. Ch3-[ch2]3-cooh
24. Chebi:17418
25. Nsc-406833
26. 64118-37-2
27. Ncgc00183281-01
28. C5:0
29. Dsstox_cid_1655
30. Dsstox_rid_76267
31. Dsstox_gsid_21655
32. Valericacid
33. Cas-109-52-4
34. Shf
35. Hsdb 5390
36. Butane-1-carboxylic Acid
37. Einecs 203-677-2
38. Unii-gzk92pjm7b
39. Mfcd00004413
40. Brn 0969454
41. Pentoate
42. Propylacetate
43. Valerianate
44. Valeriansaure
45. Ai3-08657
46. Butanecarboxylate
47. 1-pentanoate
48. 1ylv
49. 1-butanecarboxylate
50. Valeric Acid Normal
51. N-bucooh
52. 1173023-05-6
53. Valeric Acid, 99%
54. Valeric Acid, >=99%
55. Bmse000345
56. Ec 203-677-2
57. Pentanoic Acid Valeric Acid
58. Schembl5886
59. Valeric Acid [fcc]
60. Wln: Qv4
61. Valeric Acid [fhfi]
62. N-valeric Acid [mi]
63. 4-02-00-00868 (beilstein Handbook Reference)
64. Mls001066335
65. Pentanoic Acid [hsdb]
66. Valeric Acid [mart.]
67. Pentanoic Acid (valeric Acid)
68. Chembl268736
69. Gtpl1061
70. Dtxsid7021655
71. Valeric Acid ( Pentanoic Acid )
72. Valeric Acid, Analytical Standard
73. Hms2267a03
74. Valeric Acid-[3,4,5-13c3]
75. Hy-n6056
76. Tox21_113414
77. Tox21_201561
78. Tox21_303030
79. Lmfa01010005
80. Nsc406833
81. Stl169350
82. Valeric Acid, >=99%, Fcc, Fg
83. Zinc31500905
84. Akos000118960
85. Db02406
86. Ncgc00183281-02
87. Ncgc00183281-03
88. Ncgc00256597-01
89. Ncgc00259110-01
90. Bs-42203
91. Smr000471834
92. Cs-0032261
93. Ft-0651620
94. Ft-0694066
95. V0003
96. Valeric Acid, Pharmaceutical Impurity Standard
97. C00803
98. Valproic Acid Impurity A [ep Impurity]
99. Q407796
100. J-002298
101. F2191-0105
102. Z955123768
| Molecular Weight | 102.13 g/mol |
|---|---|
| Molecular Formula | C5H10O2 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 102.068079557 g/mol |
| Monoisotopic Mass | 102.068079557 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 59.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
It is metabolized by splitting into acetic acid and pyruvic acid.
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1782
Labeled valeric acid was incubated with mixed culture of cellulolytic rumen bacteria resulting in the labeling of lipids. The distribution of radioactivity indicated that 1-(14)C-labeled valeric acid was not utilized directly for biosynthesis of higher fatty acids with odd number of carbon atoms by the addition of 2 C, but it was probably degraded into 1-(14)C-labeled acetic acid and into propionic acid.
HIDIROGLOU M, LEPAGE M; CAN J BIOCHEM 45 (12): 1789 (1967)
Valeric acid is formed by rumen microorganisms during the metabolism of proline, leucine, isoleucine, norleucine, and several intermediates of carbohydrate metabolism.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:713
Valeic acid is rapidly metabolized in rat liver to acetate and propionate, giving rise to both glycogen and ketone bodies. This same route of metabolism also predominates in rumen microorganisms.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:714
For more Metabolism/Metabolites (Complete) data for n-PENTANOIC ACID (6 total), please visit the HSDB record page.
... Pentanoate was metabolized to a greater extent than octanoate and did not inhibit growth. Pentanoate inhibited acetate utilization in both the inner mitochondrial and peroxisomal compartments as indicated by a reduction in the incorporation of label from [1-14-C]acetate into lipids and into CO2, but there was no difference in oxidation of [2-14-C]pyruvate when pentanoate was the fatty acid substrate as compared to octanoate. Glyconeogenesis was inhibited when pentanoate was substituted for octanoate. ... The effects of 4-pentenoic acid were essentially the same whether octanoate or pentanoate was the fatty acid substrate, i.e. inhibition of glyconeogenesis from all labeled substrates and inhibition of [2-14-C]pyruvate oxidation.
Raugi GJ; J Biol Chem 250 (11): 4067-72 (1975)






