



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
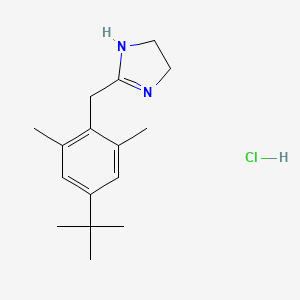

1. 2-(4'-tert-butyl-2',6'-dimethylphenylmethyl)imidazoline
2. Amidrin
3. Balkis
4. Chlorohist-la
5. Decongest
6. Espa-rhin
7. Gelonasal
8. Idasal
9. Idril N
10. Imidin
11. Nasan
12. Nasengel Al
13. Nasengel Ratiopharm
14. Nasenspray Al
15. Nasenspray Ratiopharm
16. Nasentropfen Al
17. Nasentropfen Ratiopharm
18. Novorin
19. Otradrops
20. Otraspray
21. Otriven
22. Otrivin
23. Otrivin Mentol
24. Rapako
25. Schnupfen Endrine
26. Snup
27. Stas
28. Xylometazoline
29. Xylometazoline Monohydrochloride
1. 1218-35-5
2. Xylometazoline Hcl
3. Xylometazoline (hydrochloride)
4. 2-(4-tert-butyl-2,6-dimethylbenzyl)-2-imidazoline Hydrochloride
5. Neo-synephrine Ii
6. Otrivin Hydrochloride
7. Nsc-757378
8. Mls000069695
9. Smr000058526
10. 2-(4-(tert-butyl)-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazole Hydrochloride
11. Dsstox_cid_25223
12. Dsstox_rid_80760
13. Dsstox_gsid_45223
14. 1h-imidazole, 2-[[4-(1,1-dimethylethyl)-2,6-dimethylphenyl]methyl]-4,5-dihydro-, Hydrochloride (1:1)
15. 2-(4-tert-butyl-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazole Hydrochloride
16. X5s84033nz
17. Xylometazoline Hydrochloride (usp)
18. 2-(4-tert-butyl-2,6-dimethylbenzyl)-2-imidazoline Monohydrochloride
19. 2-[(4-tert-butyl-2,6-dimethylphenyl)methyl]-4,5-dihydro-1h-imidazole;hydrochloride
20. Sr-01000000230
21. Ncgc00016101-02
22. Cas-1218-35-5
23. Mfcd00238707
24. Prestwick_574
25. Opera_id_1744
26. Neo-synephrine Ii (tn)
27. Schembl41837
28. Mls001076518
29. Spectrum1500614
30. Chembl1256400
31. Dtxsid3045223
32. Regid_for_cid_5282386
33. Hms1568n07
34. Hms1921k09
35. Pharmakon1600-01500614
36. Xylometazoline Hydrochloride ,(s)
37. Bcp22148
38. Hy-b0475
39. Tox21_110305
40. Tox21_501269
41. Ccg-39900
42. Nsc757378
43. S2576
44. Akos015895456
45. Tox21_110305_1
46. Ac-8301
47. Ks-5144
48. Lp01269
49. Ncgc00016101-10
50. Ncgc00094506-01
51. Ncgc00094506-02
52. Ncgc00094506-03
53. Ncgc00094506-04
54. Ncgc00094506-05
55. Ncgc00261954-01
56. Xylometazoline Hydrochloride [mi]
57. Xylometazoline Hydrochloride [mart.]
58. Xylometazoline Hydrochloride [vandf]
59. Eu-0101269
60. Ft-0603554
61. Sw196748-3
62. X0063
63. Xylometazoline Hydrochloride [usp-rs]
64. Xylometazoline Hydrochloride [who-dd]
65. D00757
66. X 6000
67. Xylometazoline Hydrochloride, Analytical Standard
68. 218x355
69. A804796
70. Xylometazoline Hydrochloride [ep Monograph]
71. J-004686
72. Sr-01000000230-2
73. Sr-01000000230-7
74. Xylometazoline Hydrochloride [usp Monograph]
75. Q27293585
76. Xylometazoline Hydrochloride, British Pharmacopoeia (bp) Reference Standard
77. Xylometazoline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
78. Xylometazoline Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
79. Xylometazoline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 280.83 g/mol |
|---|---|
| Molecular Formula | C16H25ClN2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 280.1706265 g/mol |
| Monoisotopic Mass | 280.1706265 g/mol |
| Topological Polar Surface Area | 24.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993) (See all compounds classified as Nasal Decongestants.)


