



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
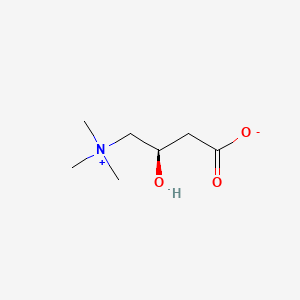

1. Bicarnesine
2. Carnitine
3. L Carnitine
4. L-carnitine
5. Vitamin Bt
1. L-carnitine
2. 541-15-1
3. (r)-carnitine
4. Vitamin Bt
5. Carnitor
6. (-)-carnitine
7. (-)-l-carnitine
8. Karnitin
9. L-(-)-carnitine
10. Carnitene
11. Levocarnitina
12. Metina
13. Carnitine, (-)-
14. L-carnitine Inner Salt
15. Levocarnitinum
16. Carniking
17. Carnilean
18. Levocarnitinum [latin]
19. Levocarnitina [spanish]
20. Carniking 50
21. (3r)-3-hydroxy-4-(trimethylammonio)butanoate
22. Gamma-trimethyl-beta-hydroxybutyrobetaine
23. Bicarnesine
24. Gamma-trimethyl-ammonium-beta-hydroxybutirate
25. 1-carnitine
26. (3r)-3-hydroxy-4-(trimethylazaniumyl)butanoate
27. 3-carboxy-2-hydroxy-n,n,n-trimethyl-1-propanaminium Hydroxide, Inner Salt
28. Drg-0211
29. Carnitine, L-
30. (r)-3-hydroxy-4-(trimethylammonio)butanoate
31. (r)-(3-carboxy-2-hydroxypropyl)trimethylammonium Hydroxide
32. 44985-71-9
33. R-(-)-3-hydroxy-4-trimethylaminobutyrate
34. (l-3-carboxy-2-hydroxypropyl)trimethylammonium Hydroxide, Inner Salt
35. 3-carboxy-2-hydroxy-n,n,n-trimethyl-1-propanaminium
36. L-gamma-trimethyl-beta-hydroxybutyrobetaine
37. Levocarnitine Hcl
38. 1-propanaminium, 3-carboxy-2-hydroxy-n,n,n-trimethyl-, Inner Salt, (r)-
39. 1-propanaminium, 3-carboxy-2-hydroxy-n,n,n-trimethyl-, Hydroxide, Inner Salt, (r)-
40. Nsc-759132
41. (3-carboxy-2-hydroxypropyl)trimethyl-ammonium Hydroxide, Inner Salt
42. Chembl1149
43. Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, Hydroxide, Inner Salt, L-
44. Carnicor
45. 0g389fzz9m
46. Carnitolo
47. Carnovis
48. Carrier
49. Chebi:16347
50. Lefcar
51. (-)-(r)-3-hydroxy-4-(trimethylammonio)butyrate
52. L-carnitine Base
53. 1-propanaminium, 3-carboxy-2-hydroxy-n,n,n-trimethyl-, Hydroxide, Inner Salt
54. 1-propanaminium, 3-carboxy-2-hydroxy-n,n,n-trimethyl-, Inner Salt, (2r)-
55. Oristar Lch
56. 1-propanaminium,3-carboxy-2-hydroxy-n,n,n-trimethyl-
57. Carnitine Hcl
58. Levocarnitine [usan:inn]
59. Vitamin B T
60. Carnitor (tn)
61. Smr000112475
62. Carnitine (l-form)
63. (r)-3-hydroxy-4-(trimethylammonio)butyrate
64. Gamma-trimethyl-hydroxybutyrobetaine
65. 3-hydroxy-4-trimethylammoniobutanoate
66. Einecs 208-768-0
67. Unii-0g389fzz9m
68. Carnipass
69. Delta-carnitine
70. Levocarnitine (jan/usp/inn)
71. L-carnitin
72. Mfcd00038747
73. Hsdb 7588
74. Carnipass 20
75. L-carnitine,(s)
76. Levocarnitine [usan:usp:inn:ban]
77. (r)-3-hydroxy-4-trimethylammoniobutyrate
78. Carnitine [mi]
79. Bmse000211
80. L-carnitine [fcc]
81. C-1985
82. L-carnitin 100 Microg/ml In Acetonitrile
83. Levocarnitine [inn]
84. Levocarnitine [jan]
85. L-carnitine (levocarnitine)
86. L-carnitine [vandf]
87. Levocarnitine [hsdb]
88. Levocarnitine [usan]
89. Schembl21970
90. Levocarnitine [vandf]
91. Mls001332549
92. Mls001332550
93. Bidd:gt0603
94. Levocarnitine [mart.]
95. Levocarnitine [usp-rs]
96. Levocarnitine [who-dd]
97. Gtpl4780
98. Dtxsid4023208
99. Hms2093j13
100. Hms2267h24
101. Pharmakon1600-01505437
102. Levocarnitine [orange Book]
103. Hy-b0399
104. Levocarnitine [ep Monograph]
105. (2r)-3-carboxy-2-hydroxy-n,n,n-trimethyl-1-propanaminium Inner Salt
106. (r)-(3-carboxy-2-hydroxypropyl)trimethylammonium Hydroxide, Inner Salt
107. Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, Hydroxide,inner Salt
108. Bdbm50037268
109. C0049
110. Levocarnitine [usp Monograph]
111. Nsc741806
112. Nsc759132
113. S2388
114. Akos005267245
115. Bcp9000830
116. Ccg-213241
117. Db00583
118. Ks-1422
119. Nsc 759132
120. Nsc-741806
121. 6-chloro-3-hydroxy(1h)indazole
122. 3-hydroxy-4-trimethylammoniobutanoic Acid
123. As-11974
124. Gamma-l-trimethyl-beta-hydroxybutyrobetaine
125. L-carnitine Inner Salt, Synthetic, >=98%
126. N1935
127. C00318
128. D02176
129. Ab00919083_05
130. Ab00919083_06
131. A829968
132. (3r)-(-)-3-hydroxy-4-(trimethylammonio)butanoate
133. Q20735709
134. (3r)-(-)-3-hydroxy-4-(trimethylammonio)butanoate 99+%
135. (3r)-3-hydroxy-4-(trimethylammonio)butanoate;l-carnitine
136. (-)-(r)-3-hydroxy-4-(trimethylammonio)butyrate, Vitamin Bt
137. Levocarnitine, European Pharmacopoeia (ep) Reference Standard
138. Levocarnitine, United States Pharmacopeia (usp) Reference Standard
139. 1-propanaminium, 3-carboxy-2-hydroxy-n,n,n-trimethyl-, Inner Salt, (2r)- (9ci)
140. Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, Hydroxide, Inner Salt, L- (8ci)
141. Levocarnitine, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 161.20 g/mol |
|---|---|
| Molecular Formula | C7H15NO3 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 161.10519334 g/mol |
| Monoisotopic Mass | 161.10519334 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 134 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Carnitor |
| PubMed Health | Levocarnitine |
| Drug Label | CARNITOR (levocarnitine) is a carrier molecule in the transport of long-chain fatty acids acrob the inner mitochondrial membrane.The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. Levocarnitin... |
| Active Ingredient | Levocarnitine |
| Dosage Form | Injectable; Tablet; Solution |
| Route | Injection; Oral |
| Strength | 330mg; 1gm/10ml; 200mg/ml |
| Market Status | Prescription |
| Company | Sigma Tau |
| 2 of 4 | |
|---|---|
| Drug Name | Levocarnitine |
| PubMed Health | Levocarnitine |
| Drug Label | Levocarnitine is a carrier molecule in the transport of long-chain fatty acids acrob the inner mitochondrial membrane.The chemical name of levocarnitine is 3-carboxy- 2(R)-hydroxy- N , N , N - t r i m e t h y l - 1 - propanaminium, inner salt. Levoc... |
| Active Ingredient | Levocarnitine |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 330mg; 1gm/10ml; 200mg/ml |
| Market Status | Prescription |
| Company | Corepharma; Teva Pharms Usa; Lyne; Hi Tech Pharma; Luitpold; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Carnitor |
| PubMed Health | Levocarnitine |
| Drug Label | CARNITOR (levocarnitine) is a carrier molecule in the transport of long-chain fatty acids acrob the inner mitochondrial membrane.The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. Levocarnitin... |
| Active Ingredient | Levocarnitine |
| Dosage Form | Injectable; Tablet; Solution |
| Route | Injection; Oral |
| Strength | 330mg; 1gm/10ml; 200mg/ml |
| Market Status | Prescription |
| Company | Sigma Tau |
| 4 of 4 | |
|---|---|
| Drug Name | Levocarnitine |
| PubMed Health | Levocarnitine |
| Drug Label | Levocarnitine is a carrier molecule in the transport of long-chain fatty acids acrob the inner mitochondrial membrane.The chemical name of levocarnitine is 3-carboxy- 2(R)-hydroxy- N , N , N - t r i m e t h y l - 1 - propanaminium, inner salt. Levoc... |
| Active Ingredient | Levocarnitine |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 330mg; 1gm/10ml; 200mg/ml |
| Market Status | Prescription |
| Company | Corepharma; Teva Pharms Usa; Lyne; Hi Tech Pharma; Luitpold; Eurohlth Intl |
Levocarnitine is indicated for treatment of primary systemic carnitine deficiency, a genetic impairment of normal biosynthesis or utilization of levocarnitine from dietary sources, or for the treatment of secondary carnitine deficiency resulting from an inborn error of metabolism. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1819
Parenteral levocarnitine is indicated for the prevention and treatment of carnitine deficiency in patients with end-stage renal disease supported on hemodialysis. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1819
Levocarnitine oral solution is used for the prevention and treatment of carnitine deficiency secondary to valproic acid toxicity. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1820
L-Carnitine, acetyl-L-carnitine, and/or propionyl-L-carnitine may be used for replacement therapy to restore normal carnitine concn and/or a normal nonesterified-to-esterified carnitine ratio ... For primary and some secondary carnitine deficiencies ... L-carnitine is used for replacement therapy.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 76 (2005)
For more Therapeutic Uses (Complete) data for L-CARNITINE (29 total), please visit the HSDB record page.
Various mild gastrointestinal complaints have been reported during the long-term administration of oral L- or D,L-carnitine; these include transient nausea and vomiting, abdominal cramps, and diarrhea. Mild myasthenia has been described only in uremic patients receiving D,L-carnitine. Gastrointestinal adverse reactions with carnitor (levocarnitine) Oral Solution or carnitor SF (levocarnitine) Sugar-Free Oral Solution dissolved in liquids might be avoided by a slow consumption of the solution or by a greater dilution. Decreasing the dosage often diminishes or eliminates drug-related patient body odor or gastrointestinal symptoms when present. Tolerance should be monitored very closely during the first week of administration, and after any dosage increases. Seizures have been reported to occur in patients with or without pre-existing seizure activity receiving either oral or intravenous levocarnitine. In patients with pre-existing seizure activity, an increase in seizure frequency and/or severity has been reported.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 3189
... Oral L-carnitine at a dose of 1 g daily was administered for twelve days to six patients with end-stage renal disease undergoing hemodialysis thrice weekly. Pre-dialysis plasma concentrations of L-carnitine (mean +/- SD) increased significantly (P < 0.05) from day 1 (baseline; 32.4 +/- 6.1 uM) to day 8 (66.1 +/- 13.8 uM) remaining constant thereafter. Although plasma levels of trimethylamine remained unaltered, the pre-dialysis plasma concentrations of trimethylamine-N-oxide increased significantly (P < 0.05) from day 1 (289.1 +/- 236.1 microM) to day 12 (529.0 +/- 237.9 uM). The hemodialysis clearances for L-carnitine, trimethylamine and trimethylamine-N-oxide were 14.3 +/- 8.2, 14.1 +/- 10.6 and 12.4 +/- 5.4 L/h, respectively, indicating their efficient removal by dialysis. Oral administration of L-carnitine at a dose of 1 g daily increases plasma concentrations of this substance to physiological levels in patients with end-stage renal disease who are undergoing hemodialysis. However, concerns about the possible deleterious consequences of such a dosage regimen still remain given that plasma concentrations of trimethylamine-N-oxide were continually rising and approximately doubled in a two-week period.
PMID:17073580 Bain MA et al; Curr Drug Metab 7 (7): 811-6 (2006)
... Patients with primary carnitine deficiency display alterations in the renal handling of L-carnitine and/or the transport of the compound into muscle tissue. Similarly, many forms of secondary carnitine deficiency, including some drug-induced disorders, arise from impaired renal tubular reabsorption. Patients with end-stage renal disease undergoing dialysis can develop a secondary carnitine deficiency due to the unrestricted loss of L-carnitine through the dialyser ...
PMID:12908852 Evans AM et al; Clin Pharmacokinet 42 (11): 941-67 (2003)
The aim of our work was to test the influence of L-carnitine supplementation on secondary hyperparathyroidism and bone metabolism in hemodialyzed patients in a randomized study. Eighty-three chronically hemodialyzed patients were observed; 44 were supplemented with L-carnitine (15 mg/kg iv after each hemodialysis for 6 months), while 39 took placebo. Levels of free carnitine (CAR), calcium (Ca), inorganic phosphate (P), Ca x P product, parathormone (PTH), bone-specific alkaline phosphatase (b-ALP), osteocalcin (OC), and osteoprotegerin (OPG) were monitored. In comparison with pretreatment values, changes of some selected parameters occurred in the supplemented patients after 6 months (data are expressed as medians; NS, nonsignificant change): PTH, 186.0 vs. 135.5 ng/L (NS); b-ALP, 13.9 vs. 13.2 ug/L (P < 0.05); OC, 78.3 vs. 68.8 ug/L (NS); OPG, 144.0 vs. 182.0 ng/L (P < 0.05). In the controls, there were the following changes: PTH, 148.0 vs. 207.0 ng/L (NS); b-ALP, 15.2 vs. 13.2 ug/L (P < 0.05); OC, 62.7 vs. 79.8 ug/L (P < 0.05); OPG, 140.0 vs. 164.0 ng/L (NS). A significant correlation was found between CAR and OPG changes (r = 0.51, P < 0.001) in the supplemented patients. The supplementation led to a significant increase of serum OPG concentration. Nevertheless, ...only nonsignificant tendencies to correction of secondary hyperparathyroidism and reduction of bone turnover in hemodialyzed patients supplemented with L-carnitine /were observed/ in contrast to controls. At this point, the use of L-carnitine does not seem to be justified.
PMID:17622482 Cibulka R et al; Calcif Tissue Int 81 (2): 99-106 (2007)
For more Drug Warnings (Complete) data for L-CARNITINE (9 total), please visit the HSDB record page.
For treatment of primary systemic carnitine deficiency, a genetic impairment of normal biosynthesis or utilization of levocarnitine from dietary sources, or for the treatment of secondary carnitine deficiency resulting from an inborn error of metabolism such as glutaric aciduria II, methyl malonic aciduria, propionic acidemia, and medium chain fatty acylCoA dehydrogenase deficiency. Used therapeutically to stimulate gastric and pancreatic secretions and in the treatment of hyperlipoproteinemias. Parenteral levocarnitine is indicated for the prevention and treatment of carnitine deficiency in patients with end-stage renal disease.
Levocarnitine is a carrier molecule in the transport of long chain fatty acids across the inner mitochondrial membrane. It also exports acyl groups from subcellular organelles and from cells to urine before they accumulate to toxic concentrations. Lack of carnitine can lead to liver, heart, and muscle problems. Carnitine deficiency is defined biochemically as abnormally low plasma concentrations of free carnitine, less than 20 µmol/L at one week post term and may be associated with low tissue and/or urine concentrations. Further, this condition may be associated with a plasma concentration ratio of acylcarnitine/levocarnitine greater than 0.4 or abnormally elevated concentrations of acylcarnitine in the urine. Only the L isomer of carnitine (sometimes called vitamin BT) affects lipid metabolism. The "vitamin BT" form actually contains D,L-carnitine, which competitively inhibits levocarnitine and can cause deficiency. Levocarnitine can be used therapeutically to stimulate gastric and pancreatic secretions and in the treatment of hyperlipoproteinemias.
A16AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AA - Amino acids and derivatives
A16AA01 - Levocarnitine
Absorption
Absolute bioavailability is 15% (tablets or solution). Time to maximum plasma concentration was found to be 3.3 hours.
Route of Elimination
Following a single intravenous dose, 73.1 +/- 16% of the dose was excreted in the urine during the 0-24 hour interval. Post administration of oral carnitine supplements, in addition to a high carnitine diet, 58-65% of the administered radioactive dose was recovered from urine and feces in 5-11 days.
Volume of Distribution
The steady state volume of distribution (Vss) of an intravenously administered dose, above endogenous baseline levels, was calculated to be 29.0 +/- 7.1L. However this value is predicted to be an underestimate of the true Vss.
Clearance
Total body clearance was found to be a mean of 4L/h.
L-Carnitine is a naturally occurring compound that facilitates the transport of fatty acids into mitochondria for beta-oxidation. ... In humans, the endogenous carnitine pool, which comprises free L-carnitine and a range of short-, medium- and long-chain esters, is maintained by absorption of L-carnitine from dietary sources, biosynthesis within the body and extensive renal tubular reabsorption from glomerular filtrate. In addition, carrier-mediated transport ensures high tissue-to-plasma concentration ratios in tissues that depend critically on fatty acid oxidation. The absorption of L-carnitine after oral administration occurs partly via carrier-mediated transport and partly by passive diffusion. After oral doses of 1-6 g, the absolute bioavailability is 5-18%. In contrast, the bioavailability of dietary L-carnitine may be as high as 75%. Therefore, pharmacological or supplemental doses of L-carnitine are absorbed less efficiently than the relatively smaller amounts present within a normal diet. L-Carnitine and its short-chain esters do not bind to plasma proteins and, although blood cells contain L-carnitine, the rate of distribution between erythrocytes and plasma is extremely slow in whole blood. After iv administration, the initial distribution volume of L-carnitine is typically about 0.2-0.3 L/kg, which corresponds to extracellular fluid volume. There are at least three distinct pharmacokinetic compartments for L-carnitine, with the slowest equilibrating pool comprising skeletal and cardiac muscle. L-Carnitine is eliminated from the body mainly via urinary excretion. Under baseline conditions, the renal clearance of L-carnitine (1-3 mL/min) is substantially less than glomerular filtration rate (GFR), indicating extensive (98-99%) tubular reabsorption. The threshold concentration for tubular reabsorption (above which the fractional reabsorption begins to decline) is about 40-60 umol/L, which is similar to the endogenous plasma L-carnitine level. Therefore, the renal clearance of L-carnitine increases after exogenous administration, approaching GFR after high iv doses. ...
PMID:12908852 Evans AM et al; Clin Pharmacokinet 42 (11): 941-67 (2003)
In mammals, the carnitine pool consists of nonesterified L-carnitine and many acylcarnitine esters. Of these esters, acetyl-L-carnitine is quantitatively and functionally the most significant. Carnitine homeostasis is maintained by absorption from diet, a modest rate of synthesis, and efficient renal reabsorption. Dietary L-carnitine is absorbed by active and passive transfer across enterocyte membranes. Bioavailability of dietary L-carnitine is 54-87% and is dependent on the amount of L-carnitine in the meal. Absorption of L-carnitine dietary supplements (0.5-6 g) is primarily passive; bioavailability is 14-18% of dose. Unabsorbed L-carnitine is mostly degraded by microorganisms in the large intestine. Circulating L-carnitine is distributed to two kinetically defined compartments: one large and slow-turnover (presumably muscle), and another relatively small and rapid-turnover (presumably liver, kidney, and other tissues). At normal dietary L-carnitine intake, whole-body turnover time in humans is 38-119 hr. In vitro experiments suggest that acetyl-L-carnitine is partially hydrolyzed in enterocytes during absorption. In vivo, circulating acetyl-L-carnitine concentration was increased 43% after oral acetyl-L-carnitine supplements of 2 g/day, indicating that acetyl-L-carnitine is absorbed at least partially without hydrolysis. After single-dose intravenous administration (0.5 g), acetyl-L-carnitine is rapidly, but not completely hydrolyzed, and acetyl-L-carnitine and L-carnitine concentrations return to baseline within 12 h. At normal circulating l-carnitine concentrations, renal l-carnitine reabsorption is highly efficient (90-99% of filtered load; clearance, 1-3 mL/min), but displays saturation kinetics. Thus, as circulating L-carnitine concentration increases (as after high-dose intravenous or oral administration of L-carnitine), efficiency of reabsorption decreases and clearance increases, resulting in rapid decline of circulating L-carnitine concentration to baseline. Elimination kinetics for acetyl-L-carnitine are similar to those for L-carnitine. There is evidence for renal tubular secretion of both L-carnitine and acetyl-L-carnitine. ...
PMID:15591001 Rebouche CJ; Ann N Y Acad Sci 1033:30-41 (2004)
The pharmacokinetics of L-carnitine and its metabolites were investigated in 7 healthy subjects following the oral administration of 0, 0.5, 1, and 2 g 3 times a day for 7 days. Mean plasma concentrations of L-carnitine across an 8-hour dose interval increased significantly (P < 0.05) from a baseline of 54.2 +/- 9.3 uM to 80.5 +/- 12.5 uM following the 0.5-g dose; there was no further increase at higher doses. There was a significant increase (P <0.001) in the renal clearance of L-carnitine indicating saturation of tubular reabsorption. Trimethylamine plasma levels increased proportionately with L-carnitine dose, but there was no change in renal clearance. A significant increase in the plasma concentrations of trimethylamine-N-oxide from baseline was evident only for the 2-g dose of L-carnitine (from 34.5 +/- 2.0 to 149 +/- 145 uM), and its renal clearance decreased with increasing dose (P <0.05). There was no evidence for nonlinearity in the metabolism of trimethylamine to trimethylamine-N-oxide. In conclusion, the pharmacokinetics of oral L-carnitine display nonlinearity above a dose of 0.5 g 3 times a day.
PMID:16988205 Bain MA et al; J Clin Pharmacol 46 (10): 1163-70 (2006)
Evidence indicates L-carnitine is absorbed in the intestine by a combination of active transport and passive diffusion. Reports of bioavailability following an oral dose have varied substantially, with estimates as low as 16 to 18% and as high as 54 to 87% ... The mucosal absorption of carnitine appears to be saturated at about a 2-g dose. Max blood concn is reached approx 3.5 hr after an oral dose and slowly decr, with a half-life of about 15 hr. Elimination of carnitine occurs primarily through the kidneys. The heart, skeletal muscle, liver, kidneys, and epididymis have specific transport systems for carnitine that concentrate carnitine within these tissues. Despite evidence indicating incr levels of free carnitine and carnitine metabolites in the blood and urine following an oral dose, no significant change in RBC carnitine levels was noted in healthy subjects, suggesting either a slow repletion of tissue stores of carnitine following an oral dose or a low capability to transport carnitine into tissues under normal conditions.
Thorne Research, Inc.; Monograph on L-Carnitine; Alternative Medicine Review 10(1) p.42 (2005). Available from, as of February 22, 2008: https://www.thorne.com/altmedrev/.fulltext/10/1/42.pdf
For more Absorption, Distribution and Excretion (Complete) data for L-CARNITINE (11 total), please visit the HSDB record page.
After oral administration L-carnitine which is unabsorbed is metabolized in the gastrointestinal tract by bacterial microflora. Major metabolites include trimethylamine N-oxide and [3H]-gamma-butyrobetaine.
In mammals, L-carnitine is synthesized from epsilon-N-trimethyllysine, which is derived from post-translationally methylated lysine residues in proteins, and protein turnover. In normal humans, the rate of synth is est to be ca 1.2 umol/kg/day. The rate of L-carnitine biosynth is regulated by the avail of epsilon-N-trimethyllysine. Thus, conditions that incr protein methylation and/or protein turnover may incr the rate of L-carnitine biosynth.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 75 (2005)
Synthesis of carnitine begins with methylation of the amino acid L-lysine by S-adenosylmethionine (SAMe). Magnesium, vitamin C, iron, vitamins B3 and B6, and alpha-ketoglutarate - along with the cofactors responsible for creating SAMe (methionine, folic acid, vitamin B12, and betaine) - are all required for endogenous carnitine synthesis.
Thorne Research, Inc.; Monograph on L-Carnitine; Alternative Medicine Review 10(1) p.42 (2005). Available from, as of February 22, 2008: https://www.thorne.com/altmedrev/.fulltext/10/1/42.pdf
Unabsorbed L-carnitine is degraded by micro-organisms in the large intestine. Major metabolites identified are trimethylamine oxide in urine and gamma-butyrobetaine in feces.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 75 (2005)
Carnitine plays an indispensable role in fatty acid oxidation. Previous studies revealed that fetal carnitine is derived from the mother via transplacental transfer. Recent studies demonstrated the presence and importance of an active fatty acid oxidation system in the human placenta and in the human fetus. In view of these findings ... carnitine metabolism /was studied/ in the fetal-placental unit by measuring carnitine metabolites, intermediary metabolites of carnitine biosynthesis, as well as the activity of carnitine biosynthesis enzymes in human term placenta, cord blood and selected embryonic and fetal tissues (5-20 weeks of development). Placenta contained low but detectable activity of gamma-butyrobetaine dioxygenase. This enzyme, which was considered to be expressed only in kidney, liver and brain, catalyzes the last step in the carnitine biosynthesis pathway. In addition, ... human fetal kidney, liver and spinal cord already have the capacity to synthesize carnitine. The ability of the placenta and fetus to synthesize carnitine suggests that in circumstances when maternal carnitine supply is limited, carnitine biosynthesis by the fetal-placental unit may supply sufficient carnitine for placental and fetal metabolism. /Carnitine/
PMID:16300828 Oey NA et al; Placenta 27 (8): 841-6 (2006)
For more Metabolism/Metabolites (Complete) data for L-CARNITINE (7 total), please visit the HSDB record page.
17.4 hours (elimination) following a single intravenous dose.
Distribution: 0.585 hours; Elimination: 17.4 hours
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1820
... Half-life /in blood/ ca 15 hr ...
Thorne Research, Inc.; Monograph on L-Carnitine; Alternative Medicine Review 10(1) p.42 (2005). Available from, as of February 22, 2008: https://www.thorne.com/altmedrev/.fulltext/10/1/42.pdf
Levocarnitine can be synthesised within the body from the amino acids lysine or methionine. Vitamin C (ascorbic acid) is essential to the synthesis of carnitine. Levocarnitine is a carrier molecule in the transport of long chain fatty acids across the inner mitochondrial membrane. It also exports acyl groups from subcellular organelles and from cells to urine before they accumulate to toxic concentrations. Only the L isomer of carnitine (sometimes called vitamin BT) affects lipid metabolism. Levocarnitine is handled by several proteins in different pathways including carnitine transporters, carnitine translocases, carnitine acetyltransferases and carnitine palmitoyltransferases.
L-Carnitine is a peripheral antagonist of thyroid hormone action in some tissues. It inhibits thyroid hormone entry into cell nuclei. In a controlled clinical trial, L-carnitine was shown to reverse or prevent some symptoms of hyperthyroidism.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 75 (2005)
... Mortality and metabolic consequences of acute ammonium intoxication in mice are reduced by pharmacologic admin of L-carnitine. The mechanism for this effect may have 2 components. L-Carnitine admin normalizes the redox state of the brain (perhaps by incr the avail of beta-hydroxybutyrate and/or acetyl-L-carnitine to the brain), and it incr the rate of urea synth in the liver, perhaps in part by activation of the glucocorticoid receptor. At least part of the protective effect is associated with flux through the carnitine acyltransferases, as analogs of L-carnitine that are competitive inhibitors of carnitine acyltransferases enhance the toxicity of acute ammonium admin. Thus, it has been proposed that L-carnitine incr urea synth in the liver by facilitating fatty acid entry into mitochondria, leading to incr flux through the beta-oxidation pathway, an incr of intramitochondrial reducing equivalents, and enhancement of ATP production. ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 78 (2005)
Levocarnitine is necessary for normal mammalian fat utilization and energy metabolism. It facilitates entry of long-chain fatty acids into cellular mitochondria, where they are used during oxidation and energy production. It also exports acyl groups from subcellular organelles and from cells to urine before they accumulate to toxic concentrations.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1820
Carnitine's primary mechanism of action is apparently attributable to its role as a cofactor in the transformation of free long-chain fatty acids into acylcarnitines for subsequent transport into the mitochondrial matrix. Carnitine is involved in the metabolism of ketones for energy and the conversion of branched-chain amino acids - valine, leucine, and isoleucine - into energy. /Carnitine/
Thorne Research, Inc.; Monograph on L-Carnitine; Alternative Medicine Review 10(1) p.43 (2005). Available from, as of February 22, 2008: https://www.thorne.com/altmedrev/.fulltext/10/1/42.pdf
L-Carnitine participates in a reversible transesterification reaction, in which an acyl group is transferred from coenzyme A to the hydroxyl group of L-carnitine ... /This reaction facilitates the/ transfer of long-chain fatty acids from cytoplasm ... /and/ chain-shortened /very-long-chain/ fatty acids from peroxisomes to mitochondria /and the/ modulation of the acyl-CoA/CoA ratio in cellular compartments.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 73 (2005)








