


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
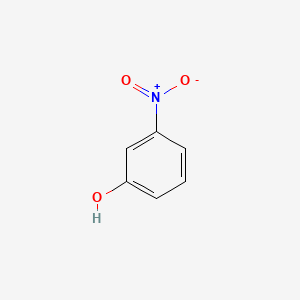

1. M-nitrophenol
1. 554-84-7
2. M-nitrophenol
3. 3-hydroxynitrobenzene
4. Phenol, 3-nitro-
5. M-hydroxynitrobenzene
6. Phenol, M-nitro-
7. M-nitrofenol
8. Meta-nitrophenol
9. 3-nitro-phenol
10. 1-hydroxy-3-nitrobenzene
11. Nsc 1551
12. T6p4t52v9w
13. Chebi:34346
14. Nsc-1551
15. M-nitrofenol [czech]
16. Crump Leather-lasting Dressing
17. Ccris 2315
18. Hsdb 1337
19. Einecs 209-073-5
20. Unii-t6p4t52v9w
21. Ai3-08825
22. 5-nitrophenol
23. 3-nitro Phenol
24. Mfcd00007240
25. Nitrophenol, 3-
26. Wln: Wnr Cq
27. Dsstox_cid_5765
28. M-nitrophenol [mi]
29. Dsstox_rid_77916
30. Dsstox_gsid_25765
31. Schembl50135
32. Mls002415747
33. 3-nitrophenol [hsdb]
34. Bidd:er0534
35. Chembl13888
36. Sr-1c3
37. 3-nitrophenol, Puriss., 99%
38. Dtxsid2025765
39. Schembl13815837
40. Nsc1551
41. Hms3039h09
42. Str00679
43. Zinc1576887
44. Tox21_200022
45. Stk290830
46. Akos000119831
47. Akos015831340
48. Akos024268500
49. M-nitrophenol [un1663] [poison]
50. 3-nitrophenol, Reagentplus(r), 99%
51. Ac-3123
52. Ncgc00090900-01
53. Ncgc00090900-02
54. Ncgc00257576-01
55. Cas-554-84-7
56. Smr001370910
57. Db-024131
58. 3-nitrophenol 100 Microg/ml In Cyclohexane
59. 3-nitrophenol, Purum, >=98.0% (hplc)
60. Ft-0616257
61. N0217
62. N-3595
63. A830666
64. Ae-562/40296193
65. Q3598793
66. Z57160187
67. F0001-1455
| Molecular Weight | 139.11 g/mol |
|---|---|
| Molecular Formula | C6H5NO3 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 139.026943022 g/mol |
| Monoisotopic Mass | 139.026943022 g/mol |
| Topological Polar Surface Area | 66 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 131 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The retention of radiolabeled 3-nitrophenol by various tissues of frog and crayfish following admin of 5 mg/kg via the lymph sac in frog and 3 mg/kg via pericardial injection in crayfish gradually decr in each organ except the gall bladder of the frog and the gut contents. In frogs, biliary excretion was minor compared with renal elimination, whereas in crayfish the gills represented the major route of excretion.
PMID:6974576 Nagel R, Urich K; Bull Environ Contam Toxicol 26 (3): 289-94 (1981)
The permeation of nitrophenols through epidermal cells from newborn rat skin cultured on type IV collagen-coated Millipore filters was studied under various conditions. The order of permeation through the cultured skin cells was found to be p-> m- > o-nitrophenol at both 10 and approx 37 C. This order was the same as that of their affinities to isolated skin cells. The permeation of nitrophenols was not inhibited by the inhibitors of energy transduction 2-deoxyglucose and NaN3. These results suggest that the permeation of nitrophenols across a cultured cell layer occurs by simple diffusion. The order of permeation of nitrophenols across newborn abdominal epidermis was exactly the opposite of that of their permeation across a cultured cell layer.
PMID:2076563 Ohkura K et al; Chem Pharm Bull (TOKYO) 38 (10):2788-91 (1990)
Yields m-aminophenol, 4-nitrocatechol, m-nitrophenyl-beta-d-glucuronide, m-nitrophenyl sulfate, & nitroquinol in rabbit.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. N-17
M-nitrophenyl-beta-d-glucoside in fly. Yields m-nitroanisole in guinea pig. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. N-17
The metabolism of nitrobenzene in male Fischer-344 rats, CD rats, and B6C3F1 mice was examined. m-Nitrophenol was one of the metabolites which was excreted as the sulfate ester by Fischer-344 rats and sulfate ester and glucuronide by CD rats. B6C3F1 mice excreted the same metabolites (except the glucuronide of m-nitrophenol) in the urine as did CD rats and also excreted p-aminophenol sulfate. In all 3 animal strains, urinary excretion of nitrobenzene metabolites peaked 12-24 hr after oral admin of nitrobenzene.
PMID:6836574 Rickert DE et al; Toxicol Appl Pharmacol 67 (2): 206-14 (1983)
In vivo studies with nitrophenols ... meta-& para-nitrophenol are reduced more readily than the ortho isomer ...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 125
For more Metabolism/Metabolites (Complete) data for 3-NITROPHENOL (10 total), please visit the HSDB record page.
Nitrophenols interfere with normal metabolism by uncoupling oxidative phosphorylation. For the mononitrophenols, the order of severity of effects is 4->3-> 2-nitrophenol.
USEPA; Nitrophenols: Hazard Profile (Draft) p.18 (1980)


