



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
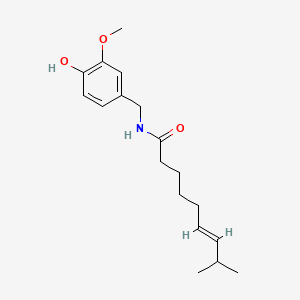

1. 8 Methyl N Vanillyl 6 Nonenamide
2. 8-methyl-n-vanillyl-6-nonenamide
3. Antiphlogistine Rub A-535 Capsaicin
4. Axsain
5. Capsaicine
6. Capsicum Farmaya
7. Capsidol
8. Capsin
9. Capzasin
10. Gelcen
11. Katrum
12. Ngx 4010
13. Ngx-4010
14. Ngx4010
15. Zacin
16. Zostrix
1. 404-86-4
2. Zostrix
3. (e)-capsaicin
4. Qutenza
5. Capsaicine
6. Axsain
7. Styptysat
8. Isodecenoic Acid Vanillylamide
9. E-capsaicin
10. Ausanil
11. Trans-capsaicin
12. Ngx-4010
13. Trans-8-methyl-n-vanillyl-6-nonenamide
14. Mioton
15. (e)-8-methyl-n-vanillyl-6-nonenamide
16. Fema No. 3404
17. Capsaicinoid
18. Algrx 4975
19. Dolenon
20. Transacin
21. Ovocap
22. 8-methyl-n-vanillyl-trans-6-nonenamide
23. Capzasin-hp
24. Togarashi Orenji
25. Nci-c56564
26. Nsc 56353
27. Ratden Pe 40
28. Chebi:3374
29. (e)-n-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
30. Capsaicin (jan/usp)
31. 6-nonenamide, 8-methyl-n-vanillyl-, (e)-
32. Ngx-1998
33. Zostrix (tn)
34. (6e)-n-(4-hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide
35. N-(4-hydroxy-3-methoxybenzyl)-8-methylnon-trans-6-enamide
36. Mfcd00017259
37. Nsc-56353
38. Cntx-4975
39. 6-nonenamide, N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-, (6e)-
40. (e)-n-(4-hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide
41. Algrx-4975
42. Chembl294199
43. S07o44r1zm
44. (6e)-n-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
45. 6-nonenamide, N-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-, (e)-
46. Nsc56353
47. Capsaicin [in Oleoresin Of Capsicum]
48. N-(4-hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide
49. 1217899-52-9
50. Trans-capsaicin-d3
51. Ncgc00017337-02
52. 8-methyl-n-vanillyl-6-nonenamide
53. 8-methyl-n-vanillyl-(trans)-6-nonenamide
54. (e)-n-((4-hydroxy-3-methoxyphenyl)-methyl)-8-methyl-6-nonenamide
55. Dsstox_cid_241
56. 6-nonenamide, (e)-n-((4-hydroxy-3-methoxy-phenyl)methyl)-8-methyl
57. 6-nonenamide, N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-, (e)-
58. Dsstox_rid_75455
59. Dsstox_gsid_20241
60. Capsaicin [usan]
61. Caswell No. 158
62. Capsicine
63. Vanilloid
64. Capsaicin (in Oleoresin Of Capsicum)
65. (6e)-n-{[4-hydroxy-3-(methyloxy)phenyl]methyl}-8-methylnon-6-enamide
66. (e)-n-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide
67. 6-nonenamide, N-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-, (6e)-
68. 7553-53-9
69. Ngx 4010
70. Ccris 1588
71. Hsdb 954
72. Sr-05000001861
73. Einecs 206-969-8
74. Epa Pesticide Chemical Code 070701
75. Brn 2816484
76. Neurotoxic
77. Unii-s07o44r1zm
78. Isodecenoate
79. N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide
80. Adlea
81. 8-methyl-n-vanillyl-6-nonenamide, (e)-
82. Tnp00277
83. (e)-n-[(4-hydroxy-3-methoxyphenyl)-methyl]-8-methyl-6-nonenamide
84. Epsilon-capsaicin
85. Capsacin(e)
86. Qutenza;vanilloid
87. Zostrix Hp
88. Capsaicin,(s)
89. 4dy
90. Cas-404-86-4
91. Prestwick_204
92. Capsaicin (natural)
93. Depletes Substance P
94. Capsaicinoids
95. Citrus Aurantium 30%
96. Starbld0019180
97. Capsaicin [usp:inn]
98. Capsaicin [inn]
99. Capsaicin [jan]
100. Capsaicin [mi]
101. Citrus Aurantium Extract
102. Isoamyl4-methoxycinnamate
103. 6-nonenamide, (e)-
104. Capsaicin [hsdb]
105. Capsaicin [inci]
106. Prestwick2_000879
107. Prestwick3_000879
108. Spectrum5_000538
109. Capsaicin [vandf]
110. Capsaicin, Natural
111. N-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-6-nonenamide, (e)-
112. Citrus Aurantium Pe
113. Capsaicin [mart.]
114. Upcmld-dp092
115. Bitter Orange Extract 30%
116. Capsaicin [usp-rs]
117. Capsaicin [who-dd]
118. Schembl8085
119. Schembl8086
120. Mr3h3
121. Bspbio_000957
122. Bspbio_001548
123. Bspbio_002917
124. Capsaicin [ema Epar]
125. Mls002154049
126. N-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-6-nonenamide
127. Capsaicin, Analytical Standard
128. Spectrum1501128
129. Bpbio1_001053
130. Gtpl2486
131. Megxp0_001448
132. Capsaicin [orange Book]
133. Dtxsid9020241
134. Methyl-n-vanillyl-6-noneneamide
135. Upcmld-dp092:001
136. Upcmld-dp092:002
137. Bdbm20461
138. Bdbm86537
139. Chebi:94524
140. Hms501b16
141. Capsaicin [usp Monograph]
142. Hms1361n10
143. Hms1570p19
144. Hms1791n10
145. Hms1921h11
146. Hms1989n10
147. Hms2089n11
148. Hms2092d21
149. Hms2097p19
150. Hms2230o23
151. Hms3402n10
152. Hms3414f11
153. Hms3649n15
154. Hms3678f11
155. Pharmakon1600-01501128
156. 8-methyl-n-vanillyl-6e-nonenamide
157. Amy25669
158. Ngx-3781
159. Ngx-7325
160. Nsc_2548
161. Zinc1530575
162. Tox21_110817
163. Tox21_200315
164. (e)-n-[(4-hydroxy-3-methoxy-phenyl)methyl]-8-methyl-non-6-enamide
165. Bbl027836
166. Ccg-39657
167. Ccg-39908
168. Ei-125
169. Hy-10448a
170. Lmfa08020085
171. N0c781
172. Nsc757844
173. S1990
174. Stl372889
175. Capsaicin 100 Microg/ml In Methanol
176. (e)8-methyl-n-vanillyl-6-nonenamide
177. Akos007930159
178. Cs-1518
179. Db06774
180. Ks-5181
181. Nsc-757844
182. Sdccgmls-0066678.p001
183. Tq-1018
184. Capsaicin 10 Microg/ml In Acetonitrile
185. Capsaicin, >=95%, From Capsicum Sp.
186. Idi1_000354
187. Idi1_034018
188. Smp2_000337
189. Ncgc00017337-03
190. Ncgc00017337-04
191. Ncgc00017337-05
192. Ncgc00017337-06
193. Ncgc00017337-07
194. Ncgc00017337-08
195. Ncgc00017337-09
196. Ncgc00017337-10
197. Ncgc00017337-11
198. Ncgc00017337-12
199. Ncgc00017337-13
200. Ncgc00017337-17
201. Ncgc00017337-18
202. Ncgc00090853-01
203. Ncgc00090853-02
204. Ncgc00090853-03
205. Ncgc00090853-04
206. Ncgc00090853-06
207. Ncgc00090853-07
208. Ncgc00090853-08
209. Ncgc00090853-09
210. Ncgc00090853-10
211. Ncgc00090853-11
212. Ncgc00090853-12
213. Ncgc00257869-01
214. Ac-10114
215. Cas_404-86-4
216. Hy-10448
217. Ls-14673
218. Smr000718774
219. Sbi-0052593.p002
220. Cs-0181240
221. M1149
222. N1667
223. (e)-8-methyl-n-vanillyl-6-nonenamide(8cl)
224. Capsaicin (8-methyl-n-vanillyl-6-nonenamide)
225. C-1700
226. C06866
227. D00250
228. Ab00053098-11
229. Ab00053098_12
230. Capsaicin, From Capsicum Sp., >=50% (hplc)
231. Capsaicin (constituent Of Capsicum) [dsc]
232. Q273169
233. Sr-05000001861-1
234. Sr-05000001861-4
235. Sr-05000001861-5
236. Sr-05000001861-6
237. Sr-05000001861-9
238. Brd-k37056290-001-01-1
239. Brd-k50590187-001-06-6
240. Capsaicin, Certified Reference Material, Tracecert(r)
241. N-(3-methoxy-4-hydroxybenzyl)-8-methyl-6-nonenamide
242. Capsaicin, European Pharmacopoeia (ep) Reference Standard
243. (6e)-n-(4-hydroxy-3-methoxybenzyl)-8-methyl-6-nonenamide #
244. Capsaicin, United States Pharmacopeia (usp) Reference Standard
245. Capsaicin; 8-methyl-n-vanillyl-trans-6-nonenamide; Ngx-4010
246. N-[(4-hydroxy-3-methoxyphenyl)methyl]-6e-8-methyl-nonenamide
247. N-(4-hydroxy-3-methoxybenzyl)-8-methyl-6-nonenamide [fhfi]
248. Trans-n-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-6-nonenamide
249. 6-nonenamide,n-((4-hydroxy-3-methoxyphenyl)methyl)-8-methyl-,(6e)-
250. Capsaicin, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 305.4 g/mol |
|---|---|
| Molecular Formula | C18H27NO3 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 9 |
| Exact Mass | 305.19909372 g/mol |
| Monoisotopic Mass | 305.19909372 g/mol |
| Topological Polar Surface Area | 58.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 341 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Qutenza |
| PubMed Health | Capsaicin (On the skin) |
| Drug Classes | Analgesic, Neuropathic Pain Agent |
| Active Ingredient | Capsaicin |
| Dosage Form | Patch |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Acorda |
| 2 of 2 | |
|---|---|
| Drug Name | Qutenza |
| PubMed Health | Capsaicin (On the skin) |
| Drug Classes | Analgesic, Neuropathic Pain Agent |
| Active Ingredient | Capsaicin |
| Dosage Form | Patch |
| Route | Topical |
| Strength | 8% |
| Market Status | Prescription |
| Company | Acorda |
Capsaicin appears to be effective for osteoarthritis (OA) pain but it is uncertain whether the effect has a dose response, is consistent across joints, or changes over time. Randomized controlled trials of topical capsaicin use in OA were identified from PubMed, EMBASE, and ISI Web of Knowledge. Effect on pain scores, patient global evaluation of treatment effectiveness and application site burning were assessed by standardised mean differences (SMD), using RevMan. Five double-blind randomized controlled trials and one case-crossover trial of topical capsaicin use were identified. Formulations ranged from 0.025 to 0.075%, and trial durations from 4 to 12 weeks. Trials assessed OA of the knee (n=3), hand (n=1), and a mix of joints (n=2). Capsaicin treatment efficacy (vs. placebo) for change in VAS pain score was moderate, at 0.44 (95% CI: 0.25-0.62) over 4 weeks of treatment. There was no heterogeneity between studies, indicating no between-study differences, including effect of OA site or treatment concentration. Two studies reported treatment beyond 4 weeks, with divergent results. One study reported an effect size of -9 mm after 12 weeks, and maximal between-group differences at 4 weeks. A second study reported that between-group differences increased over time, up to 20 weeks. Capsaicin was reported as being safe and well-tolerated, with no systemic toxicity. Mild application site burning affected 35-100% of capsaicin-treated patients with a risk ratio of 4.22 (95% CI: 3.25-5.48, n=5 trials); incidence peaked in week 1, with incidence rates declining over time. Topical capsaicin treatment four times daily is moderately effective in reducing pain intensity up to 20 weeks regardless of site of application and dose in patients with at least moderate pain and clinical or radiologically defined OA, and is well tolerated.
PMID:24941673 Laslett LL, Jones G; Prog Drug Res 68: 277-91 (2014)
Cough hypersensitivity has been common among respiratory diseases. /The study objective was/ to determine associations of capsaicin cough sensitivity and clinical parameters in adults with clinically stable bronchiectasis. We recruited 135 consecutive adult bronchiectasis patients and 22 healthy subjects. History inquiry, sputum culture, spirometry, chest high-resolution computed tomography (HRCT), Leicester Cough Questionnaire scoring, Bronchiectasis Severity Index (BSI) assessment and capsaicin inhalation challenge were performed. Cough sensitivity was measured as the capsaicin concentration eliciting at least 2 (C2) and 5 coughs (C5). Despite significant overlap between healthy subjects and bronchiectasis patients, both C2 and C5 were significantly lower in the latter group (all p<0.01). Lower levels of C5 were associated with a longer duration of bronchiectasis symptoms, worse HRCT score, higher 24-hour sputum volume, BSI and sputum purulence score, and sputum culture positive for P. aeruginosa. Determinants associated with increased capsaicin cough sensitivity, defined as C5 being 62.5 umol/L or less, encompassed female gender (OR: 3.25, 95%CI: 1.35-7.83, p<0.01), HRCT total score between 7-12 (OR: 2.57, 95%CI: 1.07-6.173, p=0.04), BSI between 5-8 (OR: 4.05, 95%CI: 1.48-11.06, p<0.01) and 9 or greater (OR: 4.38, 95%CI: 1.48-12.93, p<0.01). Capsaicin cough sensitivity is heightened in a subgroup of bronchiectasis patients and associated with the disease severity. Gender and disease severity, but not sputum purulence, are independent determinants of heightened capsaicin cough sensitivity. Current testing for cough sensitivity diagnosis may be limited because of overlap with healthy subjects but might provide an objective index for assessment of cough in future clinical trials.
PMID:25409316 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4237391 Guan WJ et al; PLoS One 9 (11): e113057 (2014)
Chronic unexplained cough triggered by environmental irritants is characterized by increased cough reflex sensitivity, which can be demonstrated by means of inhaled capsaicin. Topical capsaicin can be used to improve non-allergic rhinitis and intestinal hypersensitivity and to reduce neuropathic pain. We established whether an oral intake of natural capsaicin (chilli) could desensitize the cough reflex and improve unexplained coughing. Twenty-four patients with irritant-induced, unexplained chronic cough and 15 controls were included in the study. For 4 weeks, the participants took capsules with pure capsaicin, and for 4 weeks, they took placebo capsules. The protocol was crossover, randomized, and double blind. Cough sensitivity during the study was evaluated by a standardized capsaicin inhalation cough test that assessed the capsaicin concentration required to reach two coughs (C2) and five coughs (C5). Participants were also administered questionnaires on cough and cough-related symptoms. Three patients withdrew before the study end, one during the active treatment period and two during the placebo period. After treatment with capsaicin, the thresholds for C2 were higher (improved) both in patients (p<0.020) and in controls (p<0.0061) compared to after the placebo period. Among patients, the concentration needed to reach C2 (p<0.0004) and C5 (p<0.0009) increased after the period with the active substance compared to cough thresholds at baseline. The cough symptom scores improved after 4 weeks of active treatment (p<0.0030) compared to the baseline scores. Capsaicin powder taken orally decreased capsaicin cough sensitivity and cough symptoms. The findings suggest a desensitization of the cough-sensitive transient receptor potential vanilloid-1 (TRPV1).
PMID:25468411 Ternesten-Hasseus E et al; Respir Med 109 (1): 27-37 (2015)
Qutenza is a high-dose capsaicin patch used to relieve neuropathic pain from postherpetic neuralgia (PHN) and HIV-associated neuropathy (HIV-AN). In clinical studies, some patients had a dramatic response to the capsaicin patch. Our objective was to determine the baseline characteristics of patients who best benefit from capsaicin patch treatment. We conducted a meta-analysis of 6 completed randomized and controlled Qutenza studies by pooling individual patient data. Sustained response was defined as >50% decrease in the mean pain intensity from baseline to weeks 2 to 12, and Complete Response as an average pain intensity score=1 during weeks 2 to 12. Logistic regression was used to identify predictors of response and Complete Response, and subgroups of patients who respond best to the capsaicin patch. Baseline pain intensity score (BPIS)=4 was a predictor of Sustained and Complete Response in PHN and HIV-AN patients; absence of allodynia and presence of hypoesthesia, and a McGill Pain Questionnaire (MPQ) sensory score <22 were predictors of Sustained Response in PHN patients; female sex was a predictor of Sustained and Complete Response in HIV-AN patients. Thus, characteristics associated with the highest chance of responding to the capsaicin patch were, for PHN, BPIS=4, MPQ sensory score=22, absence of allodynia, and presence of hypoesthesia; for HIV-AN, they were female sex and BPIS=4. Patients with these characteristics had a statistically significantly greater chance of responding to the capsaicin patch than other patients.
PMID:25503598 Katz NP et al; Clin J Pain 31 (10): 859-66 (2015)
For more Therapeutic Uses (Complete) data for CAPSAICIN (21 total), please visit the HSDB record page.
A mild to moderate burning sensation is experienced following application and, in some patients, can be pronounced enough to require discontinuation of treatment.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 148
/Capsaicin must be prevented/ from entering the eyes, open lesions, or mucous membranes.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 148
... Capsaisin is for external use only. It should not be applied to wounds or to damaged or irritated skin. It should not be wrapped tightly. Capsaisin should not come in contact with mucous membranes, eyes, or contact lenses. If this occurs, the affected area should be rinsed thoroughly with water. This produc should be discontinued and a health care provider consulted if condition worsens or does not improve after regular use. If blistering occurs, or if severe burning persists. Heat should not be applied to the treated area immediately before or after applications, because this may increase the burning sensation. /Over the counter capsaicin/
Drug Facts and Comparisons 2015. Clinical Drug Information, LLC St. Louis, MO 2015, p. 3224
Do not apply prescription capsaicin to the face or scalp to avoid risk of exposure to the eyes or mucous membranes.
Drug Facts and Comparisons 2015. Clinical Drug Information, LLC St. Louis, MO 2015, p. 3224
For more Drug Warnings (Complete) data for CAPSAICIN (14 total), please visit the HSDB record page.
3(?). 3= Moderately toxic: probable oral lethal dose (human) 0.5-5 g/kg, between 1 oz & 1 pint (or 1 lb) for 70 kg person. /Capsicum/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-145
The capsaicin 8% patch is indicated in the treatment of neuropathic pain associated with post-herpetic neuralgia. There are multiple topical capsaicin formulations available, including creams and solutions, indicated for temporary analgesia in muscle and join pain as well as neuropathic pain.
FDA Label
Qutenza is indicated for the treatment of peripheral neuropathic pain in adults either alone or in combination with other medicinal products for pain.
Capsaicin is a TRPV1 receptor agonist. TRPV1 is a trans-membrane receptor-ion channel complex activated by temperatures higher than 43 degrees Celsius, pH lower than 6, and endogenous lipids. When activated by a combination of these factors, the channel can transiently open and initiate depolarization due to the influx of calcium and sodium ions. Because TRPV1 is commonly expressed in A-delta and mostly C fibers, depolarization results in action potentials which send impulses to the brain and spinal cord. These impulses result in capsaicin effects of warming, tingling, itching, stinging, or burning. Capsaicin also causes more persistent activation of these receptors compared to the environmental agonists, resulting in a loss of response to many sensory stimuli, described as "defunctionalization". Capsaicin is associated with many enzymatic, cytoskeletal, and osmotic changes, as well as disruption of mitochondrial respiration, impairing nociceptor function for extended periods of time.
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Sensory System Agents
Drugs that act on neuronal sensory receptors resulting in an increase, decrease, or modification of afferent nerve activity. (From Smith and Reynard, Textbook of Pharmacology, 1991, p367) (See all compounds classified as Sensory System Agents.)
N01BX04
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AB - Capsaicin and similar agents
M02AB01 - Capsaicin
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BX - Other local anesthetics
N01BX04 - Capsaicin
Absorption
**Oral**: Following oral administration, capsaicin may be absorbed by a nonactive process from the stomach and whole intestine with an extent of absorption ranging between 50 and 90%, depending on the animal. The peak blood concentration can be reached within 1 hour following administration. Capsaicin may undergo minor metabolism in the small intestine epithelial cells post-absorption from the stomach into the small intestines. While oral pharmacokinetics information in humans is limited, ingestion of equipotent dose of 26.6 mg of pure capsaicin, capsaicin was detected in the plasma after 10 minutes and the peak plasma concentration of 2.47 0.13 ng/ml was reached at 47.1 2.0 minutes. **Systemic**: Following intravenous or subcutaneous administration in animals, the concentrations in the brain and spinal cord were approximately 5-fold higher than that in blood and the concentration in the liver was approximately 3-fold higher than that in blood. **Topical**: Topical capsaicin in humans is rapidly and well absorbed through the skin, however systemic absorption following topical or transdermal administration is unlikely. For patients receiving the topical patch containing 179 mg of capsaicin, a population analysis was performed and plasma concentrations of capsaicin were fitted using a one-compartment model with first-order absorption and linear elimination. The mean peak plasma concentration was 1.86 ng/mL but the maximum value observed in any patient was 17.8 ng/mL.
Route of Elimination
It is proposed that capsaicin mainly undergoes renal excretion, as both the unchanged and glucuronide form. A small fraction of unchanged compound is excreted in the feces and urine. _In vivo_ animal studies demonstrates that less than 10 % of an administered dose was found in faces after 48 h.
Prescription and nonprescription products for topical management of pain, including cream, lotion and patch forms, contain capsaicin (CAP) and dihydrocapsaicin (DHC). There are few in vivo studies on absorption, bioavailability, and disposition of CAP and DHC. We established a sensitive and rapid LC-MS/MS assay to determine CAP and DHC levels in rabbit plasma and tissue. Bio-samples prepared by liquid-liquid extraction using n-hexane-dichloromethane-isopropanol (100: 50: 5, v/v/v) mixture were separated by isocratic chromatography with an Extend C18 column. The mobile phase was acetonitrile-water-formic acid (70: 30: 0.1, v/v/v). The method was linear from 0.125 to 50 ng/mL for a 100 uL bio-sample, and the lower quantification limit was 0.125 ng/mL. Total run time to analyze each sample was 3.5 min. We used this validated method to study pharmacokinetics and tissue distribution of CAP gel administered topically to rabbits. A very small amount of CAP and DHC was absorbed into the systemic circulation. The highest plasma concentration was 2.39 ng/mL, and the mean peak plasma concentration value after 12 h of CAP gel application was 1.68 ng/mL. Drug concentration in treated skin was relatively high, with low concentration in other tissues. Thus, topical CAP gel had strong local effects and weaker systemic effects.
PMID:25088519 Wang D et al; Biomed Chromatogr 29 (4): 496-503 (2015)
Capsaicin metabolism after oral administration is unclear, however it is expected to undergo metabolism in the liver with minimal metabolism in the gut lumen. _In vitro_ studies with human hepatic microsomes and S9 fragments indicate that capsaicin is rapidly metabolized, producing three major metabolites, 16-hydroxycapsaicin, 17-hydroxycapsaicin, and 16,17-hydroxycapsaicin, whereas vanillin was a minor metabolite. It is proposed that cytochrome P450 (P450) enzymes may play some role in hepatic drug metabolism. _In vitro_ studies of capsaicin in human skin suggest slow biotransformation with most capsaicin remaining unchanged.
Capsaicin and dihydrocapsaicin are the major active components in pepper spray products, which are widely used for law enforcement and self-protection. The use of pepper sprays, due to their irreversible and other health effects has been under a strong debate. In this study, we compared metabolism and cytotoxicity of capsaicin and dihydrocapsaicin using human and pig liver cell fractions and human lung carcinoma cell line (A549) in vitro. Metabolites were screened and identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Using liver cell fractions, a novel aliphatic hydroxylated metabolite (m/z 322) was detected to dihydrocapsaicin but no structure was found corresponding to capsaicin. Instead, a novel phase I metabolite of capsaicin, corresponding to the structure of aliphatic demethylation and dehydrogenation (m/z 294) was identified. In addition, two novel conjugates, glycine conjugates (m/z 363 and m/z 365) and bi-glutathione (GSH) conjugates (m/z 902 and m/z 904), were identified for both capsaicin and dihydrocapsaicin. The medium of the exposed A549 cells contained omega-hydroxylated (m/z 322) and alkyl dehydrogenated (m/z 304) forms, as well as a glycine conjugate of capsaicin. As to dihydrocapsaicin, an alkyl dehydrogenated (m/z 306) form, a novel alkyl hydroxylated form, and a novel glycine conjugate were found. In A549 cells, dihydrocapsaicin evoked vacuolization and decreased cell viability more efficiently than capsaicin. Furthermore, both compounds induced p53 protein and G1 phase cell cycle arrest. Usefulness of the found metabolites as biomarkers for capsaicinoid exposures will need further investigations with additional toxicity endpoints.
PMID:26688344 Halme M et al; J Chromatogr B Analyt Technol Biomed Life Sci 1009-1010: 17-24 (2016)
... Dehydrogenation of capsaicin was a novel metabolic pathway and produced unique macrocyclic, diene, and imide metabolites. Metabolism of capsaicin by microsomes was inhibited by 1-aminobenzotriazole (1-ABT). Metabolism was catalyzed by CYP1A1, 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4. Addition of GSH (2 mM) to microsomal incubations stimulated the metabolism of capsaicin and trapped several reactive electrophilic intermediates as their GSH adducts. /Study conducted with recombinant P450 enzymes and hepatic and lung microsomes from various species, including humans/
PMID:12641434 Reilly CA et al; Chem Res Toxicol 16 (3): 336-49 (2003)
The objectives of this study are to characterize capsaicin glucuronidation using liver microsomes and to determine the contribution of individual UDP-glucuronosyltransferase (UGT) enzymes to hepatic glucuronidation of capsaicin. The rates of glucuronidation were determined by incubating capsaicin with uridine diphosphoglucuronic acid-supplemented microsomes. Kinetic parameters were derived by model fitting. Determination of the relative activity factors, expression-activity correlation and activity correlation analysis were performed to identify the main UGT enzymes contributing to capsaicin metabolism. Capsaicin was efficiently glucuronidated in pooled human liver microsomes (pHLM). UGT1A1, 1A9 and 2B7 (as well as the gastrointestinal enzymes UGT1A7 and 1A8) showed considerable activities. Capsaicin glucuronidation was significantly correlated with 3-O-glucuronidation of beta-estradiol (r=0.637; p=0.014) and with UGT1A1 protein levels (r=0.616; p=0.019) in a bank of individual HLMs (n=14). Also, capsaicin glucuronidation was strongly correlated with zidovudine glucuronidation (r=0.765; p<0.01) and with UGT2B7 protein levels (r=0.721; p<0.01). UGT1A1, 1A9 and 2B7 contributed 30.3, 6.0 and 49.0% of total glucuronidation of capsaicin in pHLM, respectively. Further, glucuronidation of capsaicin by liver microsomes showed marked species difference.
PMID:25219630 Sun H et al; Expert Opin Drug Metab Toxicol 10 (10): 1325-36 (2014)
Following oral ingestion of equipotent dose of 26.6 mg of pure capsaicin, the half life was approximately 24.9 5.0 min. Following topical application of 3% solution of capsaicin, the half-life of capsaicin was approximately 24 h. The mean population elimination half-life was 1.64 h following application of a topical patch containing 179 mg of capsaicin.
Capsaicin has been shown to reduce the amount of substance P associated with inflammation - however this is not believed to be its main mechanism in the relief of pain. Capsaicin's mechanism of action is attributed to "defunctionalization" of nociceptor fibers by inducing a topical hypersensitivity reaction on the skin. This alteration in pain mechanisms is due to many of the following: temporary loss of membrane potential, inability to transport neurotrophic factors leading to altered phenotype, and reversible retraction of epidermal and dermal nerve fiber terminals.
Capsaicin, the pungent constituent of chili peppers, represents the paradigm for the capsaicinoids or vanilloids, a family of compounds shown to stimulate and then desensitize specific subpopulations of sensory receptors, including C-polymodal nociceptors, A-delta mechanoheat nociceptors and warm receptors of the skin, as well as enteroceptors of thin afferent fibers. ...
Szallasi A, Blumberg PM; Life Sci 47 (16): 399-40 (1990)
... In rats desensitized by intraperitoneal (ip) capsaicin (i.e., abdominal non-systemic desensitization), mainly the first but not the later fever phases were reduced. The postprandial hyperthermia to intragastric injection of BaSO4 suspension was attenuated by either ip or perineural capsaicin treatment.
PMID:16262998 Petervari E et al; J Endoxtoxin Res 11 (5): 260-6 (2005)
... Heat and protons as well as capsaicin activate VR1 to induce the influx of cations, particularly Ca2+ and Na+ ions. Characteristic effects of capsaicin are the induction of a burning sensation after acute administration and the desensitization of sensory neurons after large doses and prolonged administration. ... Capsaicin alters several visceral functions. ... Capsaicin affects thermoregulation after intra-hypothalamic injection and releases glutamate from the hypothalamus and cerebral cortex slices, while VR1-like immunoreactivity is not apparent in these regions.
PMID:10496326 Sasamura T, Kuraishi Y; Jpn J Pharmacol 80 (4): 275-80 (1999)
... 0.4 and 4 uM of capsaicin produced a significant tonic block on voltage-activated Na+ current (I(Na)) evoked by a depolarizing step to -40 mV from a holding potential of -100 mV (49 +/- 7% n=11, p<0.05 and 72 +/- 13% n=4, p<0.05 respectively). ... Capsaicin slowed the time decay of inactivation of I(Na), and increased the time constant of the recovery of inactivation. Capsaicin and tetrodotoxin (TTX) depressed contractility of isolated electrically driven left rat atria, being the depression of maximal velocity of force development (dF/dt(max)) with respect to control values of 19 +/- 3% at 1 uM of capsaicin and 22 +/- 2% at 1 uM of TTX.
PMID:11352646 Milesi V et al; Biochem Biphys Res Commun 282 (4): 965-70 (2001)
For more Mechanism of Action (Complete) data for CAPSAICIN (8 total), please visit the HSDB record page.






