



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
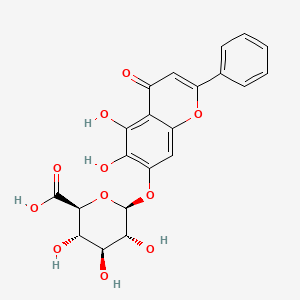

1. 7-d-glucuronic Acid-5,6-dihydroxy-flavone
2. 7-d-glucuronic Acid-5,6-dihydroxyflavone
1. 21967-41-9
2. Baicalein 7-o-glucuronide
3. 7-d-glucuronic Acid-5,6-dihydroxyflavone
4. Mfcd00134418
5. Chebi:2981
6. Chembl485818
7. 31564-28-0
8. 5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yl Beta-d-glucopyranosiduronic Acid
9. Baicalein 7-o-.beta.-d-glucuronide
10. 347q89u4m5
11. (2s,3s,4s,5r,6s)-6-((5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yl)oxy)-3,4,5-trihydroxytetrahydro-2h-pyran-2-carboxylic Acid
12. 5,6,7-trihydroxyflavone 7-o-beta-d-glucuronide
13. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenylchromen-7-yl)oxy-3,4,5-trihydroxyoxane-2-carboxylic Acid
14. (2s,3s,4s,5r,6s)-6-[(5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yl)oxy]-3,4,5-trihydroxyoxane-2-carboxylic Acid
15. 5,6-dihydroxy-4-oxo-2-phenyl-4h-1-benzopyran-7-yl
16. A-d-glucopyranosiduronic Acid
17. Baicalein 7-glucuronide
18. Baicalein-7-o-glucuronide
19. 5,6-dihydroxy-4-oxo-2-phenyl-4h-1-benzopyran-7-yl Beta-d-glucopyranosiduronic Acid
20. Baikalin
21. Baicaloside
22. Baicalin,(s)
23. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yloxy)-3,4,5-trihydroxytetrahydro-2h-pyran-2-carboxylic Acid
24. 0xe
25. Baicalin, 95%
26. Baicalin [inci]
27. Baicalin [vandf]
28. Baicalein-7-d-glucuronide
29. Baicalin [who-dd]
30. Schembl285082
31. Baicalein 7-beta-d-glucuronide
32. Baicalein 7-o-b-d-glucuronide
33. Baikal Skullcap Extract Baicalin
34. Unii-347q89u4m5
35. Tjn-151
36. Dtxsid701346569
37. Baicalin, >=99.0% (hplc)
38. Hy-n0197
39. Zinc3943903
40. Bdbm50242173
41. Akos007930529
42. Akos015955933
43. Baicalin 1000 Microg/ml In Methanol
44. Ac-7990
45. Am84780
46. Ccg-214128
47. Cs-5302
48. Beta-d-glucopyranosiduronic Acid, 5,6-dihydroxy-4-oxo-2-phenyl-4h-1-benzopyran-7-yl
49. Baicalein 7-beta-d-glucopyranosiduronate
50. Ncgc00386028-03
51. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenyl-chromen-7-yl)oxy-3,4, 5-trihydroxy-oxane-2-carboxylic Acid
52. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenyl-chromen-7-yl)oxy-3,4,5-trihydroxy-oxane-2-carboxylic Acid
53. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenyl-chromen-7-yl)oxy-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic Acid
54. As-13226
55. Baicalein 7-o-glucuronide [usp-rs]
56. 967b419
57. A815791
58. J-013512
59. Q-100275
60. Q2879368
61. Brd-k49962337-001-01-1
62. Baicalin, European Pharmacopoeia (ep) Reference Standard
63. 5,6,7-trihydroxyflavone-7-o-.beta.-d-glucopyranosideuronic Acid
64. 5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yl Ss-d-glucopyranosiduronic Acid
65. (2s,3s,4s,5r,6r)-6-(5,6-dihydroxy-4-oxo-2-phenyl-chromen-7-yl)oxy-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic Acid
66. (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4h-chromen-7-yloxy)-3,4,5-trihydroxy-tetrahydro-2h-pyran-2-carboxylic Acid
67. .beta.-d-glucopyranosiduronic Acid, 5,6-dihydroxy-4-oxo-2-phenyl-4h-1-benzopyran-7-yl
| Molecular Weight | 446.4 g/mol |
|---|---|
| Molecular Formula | C21H18O11 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 446.08491139 g/mol |
| Monoisotopic Mass | 446.08491139 g/mol |
| Topological Polar Surface Area | 183 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 748 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Anti-Asthmatic Agents
Drugs that are used to treat asthma. (See all compounds classified as Anti-Asthmatic Agents.)


