



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
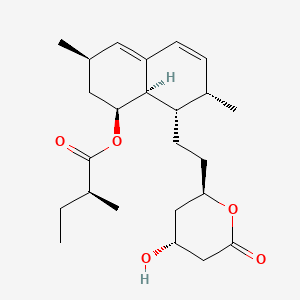

1. 1 Alpha-isomer Lovastatin
2. 6 Methylcompactin
3. 6-methylcompactin
4. Alpha-isomer Lovastatin, 1
5. Lovastatin, (1 Alpha(s*))-isomer
6. Lovastatin, 1 Alpha Isomer
7. Lovastatin, 1 Alpha-isomer
8. Mevacor
9. Mevinolin
10. Mk 803
11. Mk-803
12. Mk803
13. Monacolin K
1. 75330-75-5
2. Mevinolin
3. Mevacor
4. Altoprev
5. Mevlor
6. Sivlor
7. Mk-803
8. Monacolin K
9. Lovalord
10. Nergadan
11. Artein
12. 6alpha-methylcompactin
13. Lovalip
14. Lovastatine [french]
15. Lovastatinum [latin]
16. Lovastatina [spanish]
17. 6-alpha-methylcompactin
18. Mevinacor
19. Altocor
20. Hipovastin
21. Lovasterol
22. Cholestra
23. Closterol
24. Colevix
25. Hipolip
26. Lestatin
27. Lipivas
28. Lipofren
29. Lovastin
30. Lozutin
31. Paschol
32. Rodatin
33. Rovacor
34. Tecnolip
35. Teroltrat
36. Belvas
37. Lipdip
38. Taucor
39. Lovastatin [usan]
40. Mls000069585
41. 6.alpha.-methylcompactin
42. Msd 803
43. 9lhu78oqfd
44. [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2s)-2-methylbutanoate
45. Nsc-758662
46. L-154803
47. Mk 803
48. 2beta,6alpha-dimethyl-8alpha-(2-methyl-1-oxobutoxy)-mevinic Acid Lactone
49. Smr000058779
50. (1s,3r,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2s)-2-methylbutanoate
51. (1s,3r,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2s)-2-methylbutanoate
52. Lovastatine
53. Chebi:40303
54. C10aa02
55. Lovastatin (mevacor)
56. (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(2r,4r)-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl (s)-2-methyl-butyrate
57. Hsdb 6534
58. Ncgc00023509-03
59. Lovastatina
60. Lovastatinum
61. Dsstox_cid_784
62. Dsstox_rid_75788
63. Dsstox_gsid_20784
64. Butanoic Acid, 2-methyl-, (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl Ester, (2s)-
65. Liposcler
66. 6 Alpha-methylcompactin
67. Rextat
68. Monakolin K
69. (s)-2-methylbutyric Acid, 8-ester With (4r,6r)-6-(2-((1s,2s,6r,8s,8ar)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2h-pyran-2-one
70. (1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (s)-2-methylbutanoate
71. (s)-(1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2-methylbutanoate
72. Mevacor (tn)
73. Chembl503
74. Lovastatin & Primycin
75. Lovastatin (usp/inn)
76. Sr-05000001880
77. (1s-(1alpha(r*),3alpha,7beta,8beta(2s*,4s*),8abeta))-2-methylbutanoic Acid 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester
78. 1,2,6,7,8,8a-hexahydro-beta,delta-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobutyoxy)-1-naphthaleneheptanoic Acid Delta-lactone
79. Unii-9lhu78oqfd
80. Brn 3631989
81. Mevinolin From Aspergillus Sp.
82. Ccris 8092
83. 1cqp
84. Lovastatin,(s)
85. Ml-530b
86. Lovastatin [usan:usp:inn:ban]
87. (+)-mevinolin
88. (2s)-2-methylbutanoic Acid (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester
89. (2s)-2-methylbutanoic Acid (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl Ester
90. (s)-((1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl) 2-methylbutanoate
91. [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2s)-2-methylbutanoate
92. Lovastatin- Bio-x
93. Prestwick_819
94. Cas-75330-75-5
95. Mfcd00072164
96. Mevinolin (lovastatin)
97. Lovastatin (mevinolin)
98. Lovastatin [mi]
99. Lovastatin [inn]
100. Opera_id_1578
101. Prestwick0_000516
102. Prestwick1_000516
103. Prestwick2_000516
104. Prestwick3_000516
105. Spectrum3_001873
106. Spectrum5_001294
107. Lovastatin (mk-803)
108. Lovastatin [hsdb]
109. Lovastatin [vandf]
110. Ec 616-212-7
111. Lovastatin [mart.]
112. Schembl3136
113. Lovastatin [usp-rs]
114. Lovastatin [who-dd]
115. Us9115116, Lovastatin
116. Bidd:pxr0113
117. Bspbio_000471
118. Bspbio_001265
119. Bspbio_003346
120. Butanoic Acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1alpha(r*),3alpha,7beta,8beta(2s*,4s*),8abeta))-
121. Cid_53232
122. Mls001055358
123. Mls006011867
124. Us9353061, Lovastatina
125. Bidd:gt0749
126. Divk1c_001032
127. Spectrum1503977
128. Spbio_002392
129. Bpbio1_000519
130. Gtpl2739
131. Megxm0_000398
132. Dtxsid5020784
133. Lovastatin [orange Book]
134. Schembl14227102
135. Acon0_000534
136. Acon1_000390
137. Bdbm34168
138. Hms503o05
139. Kbio1_001032
140. Kbio3_002848
141. Lovastatin [ep Monograph]
142. Simvastatin Impurity, Lovastatin-
143. Lovastatin For Peak Identification
144. Ninds_001032
145. Hms1569h13
146. Hms1792o07
147. Hms1923o13
148. Hms1990o07
149. Hms2089m06
150. Hms2093o03
151. Hms2096h13
152. Hms2236f07
153. Hms3039n16
154. Hms3259f10
155. Hms3268c03
156. Hms3403o07
157. Hms3412h19
158. Hms3676h19
159. Hms3713h13
160. Hms3884b03
161. Lovastatin [usp Monograph]
162. Pharmakon1600-01503977
163. Advicor Component Lovastatin
164. Act02620
165. Albb-027272
166. Butanoic Acid, 2-methyl-, (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (2s)-
167. Hy-n0504
168. Mk-803; Lovalip; Mevacor
169. Zinc3812841
170. Tox21_110888
171. Tox21_201475
172. Tox21_300268
173. Bbl024473
174. Ccg-39627
175. Nsc633781
176. Nsc758662
177. Nsc779704
178. S2061
179. Stk801953
180. Akos005267139
181. Lovastatin Component Of Advicor
182. Tox21_110888_1
183. Cs-1990
184. Db00227
185. Ks-1082
186. Mevinolin From Aspergillus Sp., Powder
187. Nc00713
188. Nsc 758662
189. Nsc-633781
190. Nsc-779704
191. Idi1_001032
192. Ncgc00023509-04
193. Ncgc00023509-05
194. Ncgc00023509-06
195. Ncgc00023509-07
196. Ncgc00023509-08
197. Ncgc00023509-09
198. Ncgc00023509-10
199. Ncgc00023509-11
200. Ncgc00023509-13
201. Ncgc00023509-14
202. Ncgc00023509-16
203. Ncgc00254157-01
204. Ncgc00259026-01
205. 74133-25-8
206. Ac-13961
207. Bl164644
208. Butanoic Acid, 2-methyl-, (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (2s)-
209. G226
210. Smr000673570
211. Sbi-0051881.p002
212. L0214
213. N1632
214. Simvastatin Impurity E [ep Impurity]
215. En300-52515
216. C07074
217. D00359
218. Ab00052400-17
219. Ab00052400_18
220. Ab00052400_19
221. Mevinolin From Aspergillus Sp., >=98% (hplc)
222. 330l755
223. A838030
224. A838383
225. Q417740
226. Sr-01000000123
227. Sr-01000000123-3
228. Sr-05000001880-1
229. Sr-05000001880-2
230. Brd-k09416995-001-06-8
231. Brd-k09416995-001-21-7
232. Mevacor;monacolin K;mevinolin;6-alpha-methylcompactin
233. Simvastatin Impurity, Lovastatin- [usp Impurity]
234. Z1258578375
235. (1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-8-(2-((4r,6r)-4-hydroxy-2-oxo-2h-pyran-6-yl)ethyl)-3,7-dimethylnaphtyl(s)-2-methylbutyrat
236. (2s)-(1s,3r,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl-2-methyl Butanoate
237. (2s)-2-methylbutanoic Acid [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxo-2-oxanyl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] Ester
238. (s)-((1s,3r,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxo-tetrahydro-2h-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl) 2-methylbutanoate
239. (s)-2-methyl-butyric Acid (1s,3r,7s,8s,8ar)-8-[2-((3r,5r)-4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl Ester
240. [(1s,3r,7s,8s,8ar)-3,7-dimethyl-8-[2-[(2r,4r)-4-oxidanyl-6-oxidanylidene-oxan-2-yl]ethyl]-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2s)-2-methylbutanoate
241. [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2-methylbutanoate
242. 1s,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2s)-2-methylbutanoate
243. 8-[2-(4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthale
244. Butanoic Acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-- Oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1alpha(r*),3alpha,7beta,8beta(2s*,4s*),8abeta))-
245. Butanoic Acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1.alpha.(r*),3.alpha.,7.beta.,8.beta.(2s*,4s*),8.alpha..beta.))-
| Molecular Weight | 404.5 g/mol |
|---|---|
| Molecular Formula | C24H36O5 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 404.25627424 g/mol |
| Monoisotopic Mass | 404.25627424 g/mol |
| Topological Polar Surface Area | 72.8 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 666 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Altoprev |
| PubMed Health | Lovastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Altoprev lovastatin extended-release tablets contain a cholesterol-lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the corresponding -hydroxyacid form.... |
| Active Ingredient | Lovastatin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 60mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Andrx Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Lovastatin |
| PubMed Health | Lovastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Lovastatin is a cholesterol lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the correspondingform. This is a principal metabolite and an inhibitor of 3-hyd... |
| Active Ingredient | Lovastatin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Teva; Apotex; Lupin; Sandoz; Actavis Elizabeth; Mutual Pharm; Carlsbad; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Altoprev |
| PubMed Health | Lovastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Altoprev lovastatin extended-release tablets contain a cholesterol-lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the corresponding -hydroxyacid form.... |
| Active Ingredient | Lovastatin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 60mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Andrx Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Lovastatin |
| PubMed Health | Lovastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | Lovastatin is a cholesterol lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the correspondingform. This is a principal metabolite and an inhibitor of 3-hyd... |
| Active Ingredient | Lovastatin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Teva; Apotex; Lupin; Sandoz; Actavis Elizabeth; Mutual Pharm; Carlsbad; Mylan |
Anticholesteremic Agents; Hydroxymethylglutaryl-CoA Reductase Inhibitors
National Library of Medicine's Medical Subject Headings. Lovastatin. Online file (MeSH, 2016). Available from, as of November 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lovastatin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=LOVASTATIN&Search=Search
Lovastatin Tablets USP are indicated as an adjunct to diet to reduce total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B levels in adolescent boys and girls who are at least one year post-menarche, 10 to 17 years of age, with heFH if after an adequate trial of diet therapy the following findings are present: 1. LDL-C remains > 189 mg/dL or 2. LDL-C remains > 160 mg/dL and: there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the adolescent patient. /Included in US product label/
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Lovastatin Tablets USP are indicated as an adjunct to diet for the reduction of elevated total cholesterol (total-C) and low-density lipoprotein cholesterol (LDL-C) levels in patients with primary hypercholesterolemia (Types IIa and IIb), when the response to diet restricted in saturated fat and cholesterol and to other nonpharmacological measures alone has been inadequate. /Included in US product label/
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
For more Therapeutic Uses (Complete) data for Lovastatin (9 total), please visit the HSDB record page.
Lovastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Lovastatin is contraindicated in women who are or may become pregnant. The drug should be administered to women of childbearing age only when such patients are highly unlikely to conceive and have been informed of the potential hazard. If the patient becomes pregnant while receiving lovastatin, the drug should be discontinued immediately and the patient informed of the potential hazard to the fetus.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1854
Maternal treatment with lovastatin may reduce the fetal levels of mevalonate, which is a precursor of cholesterol biosynthesis. Atherosclerosis is a chronic process, and ordinarily discontinuation of lipid-lowering drugs during pregnancy should have little impact on the long-term risk associated with primary hypercholesterolemia. For these reasons, lovastatin should not be used in women who are pregnant, or can become pregnant. Lovastatin should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. Treatment should be immediately discontinued as soon as pregnancy is recognized.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including lovastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with lovastatin, promptly interrupt therapy. If an alternate etiology is not found, do not restart lovastatin.
Health Canada; Product Monograph for Lovastatin (20 mg and 40 mg Tablets USP), Drug Identification Number (DIN): 02344335 p.7 (Date of Revision: May 29, 2014). Available from, as of February 1, 2017: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
For more Drug Warnings (Complete) data for Lovastatin (30 total), please visit the HSDB record page.
Lovastatin is indicated to reduce the risk of myocardial infarction, unstable angina, and the need for coronary revascularization procedures in individuals without symptomatic cardiovascular disease, average to moderately elevated total-C and LDL-C, and below average HDL-C. It is indicated as an intervention alternative in individuals presenting dyslipidemia at risk of developing atherosclerotic vascular disease. The administration of this agent should be accompanied by the implementation of a fat and cholesterol-restricted diet. Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Lovastatin is indicated as an adjunct to diet for the reduction of elevated total-C and LDL-C levels in patients with primary hypercholesterolemia (Types IIa and IIb2), when the response to diet restricted in saturated fat and cholesterol and to other nonpharmacological measures alone has been inadequate. Lovastatin is also indicated to slow the progression of coronary atherosclerosis in patients with coronary heart disease as part of a treatment strategy to lower total-C and LDL-C to target levels. Lovastatin is indicated as an adjunct to diet to reduce total-C, LDL-C and apolipoprotein B levels in adolescent boys and girls with Heterozygous Familial Hypercholesterolemia (HeFH) who are at least one year post-menarche, 10 to 17 years of age, with HeFH if after an adequate trial of diet therapy the following findings are present: LDL-C remains greater than 189 mg/dL or LDL-C remains greater than 160 mg/dL and there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the adolescent patient. Before administering lovastatin, it is important to rule out the presence of secondary causes of hypercholesterolemia and a lipid profile should be performed. Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Lovastatin is an oral antilipemic agent which reversibly inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, lovastatin reduces the risk of cardiovascular morbidity and mortality. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. Clinical studies have shown that lovastatin reduces LDL-C and total cholesterol by 25-40%. The 50% inhibitory dose is known to be of 46 mcg/kg which is translated into a reduction of approximately 30% of plasma cholesterol. **Myopathy/Rhabdomyolysis** Lovastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is dose-related and is increased by high levels of HMG-CoA reductase inhibitory activity in plasma. In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20 to 40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily. Predisposing factors for myopathy include advanced age (65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may also be at increased risk for myopathy. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. The risk of myopathy during treatment with lovastatin may be increased with concurrent administration of interacting drugs such as [fenofibrate], [niacin], [gemfibrozil], [cyclosporine], and strong inhibitors of the CYP3A4 enzyme. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with [colchicine], and caution should therefore be exercised when prescribing these two medications together. Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment. **Liver Dysfunction** Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials. When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. In the EXCEL study, the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages.
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AA - Hmg coa reductase inhibitors
C10AA02 - Lovastatin
Absorption
Lovastatin Cmax was found to be 3.013ng/mL with a Tmax of 3.36 hours. Plasma concentrations of total radioactivity (lovastatin plus 14C-metabolites) peaked at 2 hours and declined rapidly to about 10% of peak by 24 hours postdose. Absorption of lovastatin, estimated relative to an intravenous reference dose, in each of four animal species tested, averaged about 30% of an oral dose. In animal studies, after oral dosing, lovastatin had high selectivity for the liver, where it achieved substantially higher concentrations than in non-target tissues. Lovastatin undergoes extensive first-pass extraction in the liver, its primary site of action, with subsequent excretion of drug equivalents in the bile. As a consequence of extensive hepatic extraction of lovastatin, the availability of drug to the general circulation is low and variable. In a single dose study in four hypercholesterolemic patients, it was estimated that less than 5% of an oral dose of lovastatin reaches the general circulation as active inhibitors. Following administration of lovastatin tablets the coefficient of variation, based on between-subject variability, was approximately 40% for the area under the curve (AUC) of total inhibitory activity in the general circulation. The peak concentrations of lovastatin when a dose of 10-40 mg is administered are reported to range from 1.04-4.03 ng/ml and an AUC of 14-53 ng.h/ml. This indicates that lovastatin presents a dose-dependent pharmacokinetic profile. When lovastatin was given under fasting conditions, plasma concentrations of both active and total inhibitors were on average about two-thirds those found when lovastatin was administered immediately after a standard test meal. Genetic differences in the OATP1B1 (Organic-Anion-Transporting Polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact lovastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) showed that lovastatin Cmax and AUC were 340 and 286% higher, respectively, for individuals homozygous for 521CC compared to homozygous 521TT individuals. The 521CC genotype is also associated with a marked increase in the risk of developing myopathy, likely secondary to increased systemic exposure. Other statin drugs impacted by this polymorphism include [rosuvastatin], [pitavastatin], [atorvastatin], [simvastatin], and [pravastatin]. While specific dosage instructions are not included in the available product monographs for lovastatin, individuals with the above-mentioned c.521CC OATP1B1 genotype should be monitored for development of adverse effects from increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis, particularly at higher doses.
Route of Elimination
Following an oral dose of 14C-labeled lovastatin to man, 10% of the dose was excreted in urine and 83% in feces. The latter represents absorbed drug excreted in bile, together with unabsorbed drug.
Volume of Distribution
Lovastatin is able to cross the blood-brain barrier and placenta.
/MILK/ It is not known whether lovastatin is excreted in human milk.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Following an oral dose of (14)C-labeled lovastatin in man, 10% of the dose was excreted in urine and 83% in feces. The latter represents absorbed drug equivalents excreted in bile, as well as any unabsorbed drug. Plasma concentrations of total radioactivity (lovastatin plus (14)C-metabolites) peaked at 2 hours and declined rapidly to about 10% of peak by 24 hours postdose. Absorption of lovastatin, estimated relative to an intravenous reference dose, in each of four animal species tested, averaged about 30% of an oral dose. In animal studies, after oral dosing, lovastatin had high selectivity for the liver, where it achieved substantially higher concentrations than in non-target tissues. Lovastatin undergoes extensive first-pass extraction in the liver, its primary site of action, with subsequent excretion of drug equivalents in the bile. As a consequence of extensive hepatic extraction of lovastatin, the availability of drug to the general circulation is low and variable. In a single dose study in four hypercholesterolemic patients, it was estimated that less than 5% of an oral dose of lovastatin reaches the general circulation as active inhibitors. Following administration of lovastatin tablets the coefficient of variation, based on between-subject variability, was approximately 40% for the area under the curve (AUC) of total inhibitory activity in the general circulation.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Both lovastatin and its beta-hydroxyacid metabolite are highly bound (> 95%) to human plasma proteins. Animal studies demonstrated that lovastatin crosses the blood-brain and placental barriers.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Peak plasma concentrations of both active and total inhibitors were attained within 2 to 4 hours of dose administration.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Lovastatin is given as a lactone prodrug and thus, in order to produce its mechanism of action, it is required to be converted to the active beta-hydroxy form. This drug activation process does not seem to be related to CYP isoenzyme activity but rather to be controlled by the activity of serum paraoxonase. Lovastatin is metabolized by the microsomal hepatic enzyme system (Cytochrome P-450 isoform 3A4). The major active metabolites present in human plasma are the -hydroxy acid of lovastatin, its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. The uptake of lovastatin by the liver is enhanced by the activity of OATP1B1.
Lovastatin is metabolized by the microsomal hepatic enzyme system (Cytochrome P-450 isoform 3A4). The major active metabolites present in human plasma are the beta-hydroxy acid of lovastatin, its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives.
Health Canada; Product Monograph for Lovastatin (20 mg and 40 mg Tablets USP), Drug Identification Number (DIN): 02344335 p.28 (Date of Revision: May 29, 2014). Available from, as of February 1, 2017: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
The major active metabolites present in human plasma are the beta-hydroxyacid of lovastatin, its 6'-hydroxy derivative, and two additional metabolites.
NIH; DailyMed. Current Medication Information for Lovastatin (Lovastatin Tablet) (Updated: June 2016). Available from, as of February 1, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e15feec-d27e-4861-8152-c5a8b8ccacd4
Lovastatin has known human metabolites that include 3-Hydroxylovastatin and 6'beta-Hydroxylovastatin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Lovastatin half-life is reported to be of 13.37 hours. The elimination half-life of the hydroxy acid form of lovastatin is reported to be of 0.7-3 hours.
Lovastatin is a lactone which is readily hydrolyzed _in vivo_ to the corresponding -hydroxyacid and strong inhibitor of HMG-CoA reductase, a hepatic microsomal enzyme which catalyzes the conversion of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A ) to mevalonate, an early rate-limiting step in cholesterol biosynthesis. At therapeutic lovastatin doses, HMG-CoA reductase is not completely blocked, thereby allowing biologically necessary amounts of mevalonate to be available. Because the conversion of HMG-CoA to mevalonate is an early step in the biosynthetic pathway for cholesterol, therapy with lovastatin would not be expected to cause an accumulation of potentially toxic sterols. Lovastatin acts primarily in the liver, where decreased hepatic cholesterol concentrations stimulate the upregulation of hepatic low density lipoprotein (LDL) receptors which increase hepatic uptake of LDL. Lovastatin also inhibits hepatic synthesis of very low density lipoprotein (VLDL). The overall effect is a decrease in plasma LDL and VLDL and a significant reduction in the risk of development of CVD and all-cause mortality. A significant effect on LDL-C reduction was seen within 2 weeks of initiation of lovastatin, and the maximum therapeutic response occurred within 4-6 weeks. The response was maintained during continuation of therapy. Single daily doses given in the evening were more effective than the same dose given in the morning, perhaps because cholesterol is synthesized mainly at night. When therapy with lovastatin is stopped, total cholesterol has been shown to return to pre-treatment levels. In vitro and in vivo animal studies also demonstrate that lovastatin exerts vasculoprotective effects independent of its lipid-lowering properties, also known as the pleiotropic effects of statins. This includes improvement in endothelial function, enhanced stability of atherosclerotic plaques, reduced oxidative stress and inflammation, and inhibition of the thrombogenic response. Statins have also been found to bind allosterically to 2 integrin function-associated antigen-1 (LFA-1), which plays an important role in leukocyte trafficking and in T cell activation. Lovastatin has been reported to have beneficial effects on certain cancers. This includes a multi-factorial stress-triggered cell death (apoptosis) and DNA degradation response in breast cancer cells. It has also been shown to inhibit histone deacetylase 2 (HDAC2) activity and increase the accumulation of acetylated histone-H3 and the expression of p21(WAF/CIP) in human cancer cells, suggesting that statins might serve as novel HDAC inhibitors for cancer therapy and chemoprevention.
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins), atorvastatin, cerivastatin, fluvastatin, pravastatin, lovastatin and simvastatin, reduce atherogenesis and cardiovascular morbidity. Besides, there is growing evidence that statins have immunomodulatory activities. Statins downregulate the expression of adhesion molecules, intercellular adhesion molecule-1 (ICAM-1), monocyte chemotactic protein-1 (MAC-1) and lymphocyte function-associated antigen-1 (LFA-1), on leucocytes and endothelial cells and, through binding to LFA-1, interfere with ICAM-1-LFA-1 interaction, which is crucial for activation of lymphocytes by antigen-presenting cells, ingress of leucocytes into the inflammation sites and immunologic cytotoxicity. Statins inhibit the inducible expression of major histocompatibility complex class II in several cell types including macrophages and downregulate the expression of T-helper-1 (Th1) chemokine receptors on T cells, leading further to inhibition of activation of lymphocytes and their infiltration into the inflammation sites. Statins block the induction of inducible nitric oxide synthase and the expression of several proinflammatory cytokines such as tumor necrosis factor-alpha and interferon-gamma in macrophages and possess antioxidant effects. These agents inhibit the proliferation of immunocytes and the activation of natural killer cells.
PMID:15186318 Namazi MR; Exp Dermatol 13 (6): 337-9 (2004)
Lovastatin, is a cholesterol-lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the corresponding beta-hydroxy acid form. This principal metabolite is a specific inhibitor of 3-hydroxy-3- methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and ratelimiting step in the biosynthesis of cholesterol.
Health Canada; Product Monograph for Lovastatin (20 mg and 40 mg Tablets USP), Drug Identification Number (DIN): 02344335 p.20 (Date of Revision: May 29, 2014). Available from, as of February 1, 2017: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
Lovastatin is a member of Statins, which are beneficial in a lot of immunologic cardiovascular diseases and T cell-mediated autoimmune diseases. Kv1.3 channel plays important roles in the activation and proliferation of T cells, and have become attractive target for immune-related disorders. The present study was designed to examine the block effect of Lovastatin on Kv1.3 channel in human T cells, and to clarify its new immunomodulatory mechanism. We found that Lovastatin inhibited Kv1.3 currents in a concentration- and voltage-dependent manner, and the IC50 for peak, end of the pulse was 39.81 +/- 5.11, 6.92 +/- 0.95 uM, respectively. Lovastatin also accelerated the decay rate of current inactivation and negatively shifted the steady-state inactivation curves concentration-dependently, without affecting the activation curve. However, 30 uM Lovastatin had no apparent effect on KCa current in human T cells. Furthermore, Lovastatin inhibited Ca(2+) influx, T cell proliferation as well as IL-2 production. The activities of NFAT1 and NF-kB p65/50 were down-regulated by Lovastatin, too. At last, Mevalonate application only partially reversed the inhibition of Lovastatin on IL-2 secretion, and the siRNA against Kv1.3 also partially reduced this inhibitory effect of Lovastatin. In conclusion, Lovastatin can exert immunodulatory properties through the new mechanism of blocking Kv1.3 channel.
PMID:26616555 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4663632 Zhao N et al; Sci Rep. 2015 Nov 30;5:17381. doi: 10.1038/srep17381








