



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0

1. A 64077
2. A-64077
3. Abbot 64077
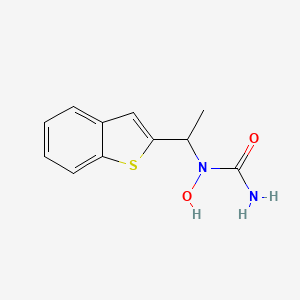
4. N-(1-benzo(b)thien-2-ylethyl)-n-hydroxyurea
5. Zyflo
1. 111406-87-2
2. Zyflo
3. Leutrol
4. 1-(1-(benzo[b]thiophen-2-yl)ethyl)-1-hydroxyurea
5. Zileutonum
6. Abbott 64077
7. Zileutonum [inn-latin]
8. 1-[1-(1-benzothiophen-2-yl)ethyl]-1-hydroxyurea
9. A-64077
10. N-(1-benzo(b)thien-2-ylethyl)-n-hydroxyurea
11. A 64077
12. Zyflo (tn)
13. Abbott-64077
14. Urea, N-(1-benzo[b]thien-2-ylethyl)-n-hydroxy-
15. Chembl93
16. 1-[1-(1-benzothien-2-yl)ethyl]-1-hydroxyurea
17. Nsc-730712
18. Nsc-759277
19. N-[1-(benzo[b]thiophen-2-yl)ethyl]-n-hydroxyurea
20. V1l22wve2s
21. (+-)-1-(1-benzo(b)thien-2-ylethyl)-1-hydroxyurea
22. (+/-)-1-(1-benzo[b]thien-2-ylethyl)-1-hydroxyurea
23. Zyflo Cr
24. Chebi:10112
25. N-(1-benzo[b]thien-2-ylethyl)-n-hydroxyurea
26. (+-)-1-(1-benzo[b]thien-2-ylethyl)-1-hydroxyurea
27. N-(1-benzo(b)thien-2-yl-ethyl)-n-hydroxyurea
28. Ncgc00159453-02
29. Dsstox_cid_3752
30. Dsstox_rid_77185
31. Dsstox_gsid_23752
32. Urea, N-(1-benzo(b)thien-2-ylethyl)-n-hydroxy-
33. Ziluton
34. Zyflo Filmtab
35. ( Inverted Exclamation Marka)-1(1-benzo[b]thien-2-ylethyl)-1-hydroxyure
36. Smr000466377
37. Cas-111406-87-2
38. Zileuton (usp/inn)
39. Sr-01000759349
40. (+/-)-1-(1-benzo(b)thien-2-ylethyl)-1-hydroxyurea
41. Unii-v1l22wve2s
42. Cti-02
43. Abt-077
44. Zileuton [usan:usp:inn:ban]
45. Zileuton- Bio-x
46. Mfcd00866097
47. Starbld0016861
48. Zileuton [usan]
49. Zileuton [inn]
50. Zileuton [mi]
51. Zileuton [vandf]
52. Prestwick0_001090
53. Zileuton [mart.]
54. Zileuton [usp-rs]
55. Zileuton [who-dd]
56. Schembl4209
57. Mls000759510
58. Mls001424079
59. Mls006011971
60. Zileuton [orange Book]
61. Gtpl5297
62. Zileuton, >=98% (hplc)
63. Dtxsid9023752
64. Schembl18251470
65. Zileuton [usp Monograph]
66. Urea, N-(1-benzo(b)thien-2-ylethyl)-n-hydroxy-, (+-)-
67. Hms2051m20
68. Hms2089j12
69. Hms2093h06
70. Hms2235o04
71. Hms3369i17
72. Hms3393m20
73. Hms3654j08
74. Hms3714e07
75. Hms3872f13
76. Pharmakon1600-01505906
77. Amy12540
78. Bcp16199
79. Tox21_111680
80. Tox21_301148
81. Bbl029070
82. Bdbm50000541
83. Nsc730712
84. Nsc759277
85. S1443
86. Stl373010
87. Akos000280127
88. Akos016340558
89. Tox21_111680_1
90. Ccg-100901
91. Ccg-213571
92. Cs-1563
93. Db00744
94. Ks-1195
95. Nc00151
96. Nsc 730712
97. Nsc 759277
98. Sb19083
99. Mrf-0000030
100. Ncgc00159453-03
101. Ncgc00159453-04
102. Ncgc00159453-05
103. Ncgc00159453-06
104. Ncgc00255046-01
105. Ac-13198
106. Ac-31490
107. Bz164573
108. Hy-14164
109. Sbi-0206869.p001
110. Db-015071
111. Bb 0261152
112. Ft-0601582
113. Ft-0675903
114. Sw197531-3
115. 1-(1-benzothiophen-2-ylethyl)-1-hydroxy-urea
116. D00414
117. N-(1-benzo[b]thien-2-ylethyl)-n-hydroxy-urea
118. Ab00639921-06
119. Ab00639921-08
120. Ab00639921-09
121. Ab00639921_10
122. Ab00639921_11
123. 406z872
124. A802357
125. Q202998
126. J-002574
127. J-525169
128. Sr-01000759349-4
129. Sr-01000759349-5
130. Sr-01000759349-6
131. Zileuton, Commercially Available As 600 Mg Tablets
132. Brd-a56359832-001-04-6
133. Z1551429725
134. Zileuton, United States Pharmacopeia (usp) Reference Standard
135. Urea, N-(1-benzo(b)thien-2-ylethyl)-n-hydroxy-, (+/-)-
| Molecular Weight | 236.29 g/mol |
|---|---|
| Molecular Formula | C11H12N2O2S |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 236.06194880 g/mol |
| Monoisotopic Mass | 236.06194880 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 275 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Zyflo |
| PubMed Health | Zileuton (By mouth) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Zileuton is an orally active inhibitor of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Zileuton has the chemical name ()-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea and the following chemical struct... |
| Active Ingredient | Zileuton |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Chiesi Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Zyflo cr |
| PubMed Health | Zileuton (By mouth) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Zileuton is an orally active inhibitor of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Zileuton has the chemical name ()-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea and the following chemical struct... |
| Active Ingredient | Zileuton |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Chiesi Usa |
| 3 of 4 | |
|---|---|
| Drug Name | Zyflo |
| PubMed Health | Zileuton (By mouth) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Zileuton is an orally active inhibitor of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Zileuton has the chemical name ()-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea and the following chemical struct... |
| Active Ingredient | Zileuton |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Chiesi Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Zyflo cr |
| PubMed Health | Zileuton (By mouth) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Zileuton is an orally active inhibitor of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Zileuton has the chemical name ()-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea and the following chemical struct... |
| Active Ingredient | Zileuton |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg |
| Market Status | Prescription |
| Company | Chiesi Usa |
For the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older.
FDA Label
Zileuton is an asthma drug that differs chemically and pharmacologically from other antiasthmatic agents. It blocks leukotriene synthesis by inhibiting 5-lipoxygenase, an enzyme of the eicosanoid synthesis pathway. Current data indicates that asthma is a chronic inflammatory disorder of the airways involving the production and activity of several endogenous inflammatory mediators, including leukotrienes. Sulfido-peptide leukotrienes (LTC4, LTD4, LTE4, also known as the slow-releasing substances of anaphylaxis) and LTB4, a chemoattractant for neutrophils and eosinophils, are derived from the initial unstable product of arachidonic acid metabolism, leukotriene A4 (LTA4), and can be measured in a number of biological fluids including bronchoalveolar lavage fluid (BALF) from asthmatic patients. In humans, pretreatment with zileuton attenuated bronchoconstriction caused by cold air challenge in patients with asthma.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Lipoxygenase Inhibitors
Compounds that bind to and inhibit that enzymatic activity of LIPOXYGENASES. Included under this category are inhibitors that are specific for lipoxygenase subtypes and act to reduce the production of LEUKOTRIENES. (See all compounds classified as Lipoxygenase Inhibitors.)
Leukotriene Antagonists
A class of drugs designed to prevent leukotriene synthesis or activity by blocking binding at the receptor level. (See all compounds classified as Leukotriene Antagonists.)
Absorption
Rapidly and almost completely absorbed. The absolute bioavailability is unknown.
Route of Elimination
Elimination of zileuton is predominantly via metabolism with a mean terminal half-life of 2.5 hours. The urinary excretion of the inactive N-dehydroxylated metabolite and unchanged zileuton each accounted for less than 0.5% of the dose.
Volume of Distribution
1.2 L/kg
Clearance
Apparent oral cl=7 mL/min/kg
Hepatic. Zileuton and its N-dehydroxylated metabolite are oxidatively metabolized by the cytochrome P450 isoenzymes 1A2, 2C9 and 3A4.
Zileuton has known human metabolites that include Zileuton O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
2.5 hours
Leukotrienes are substances that induce numerous biological effects including augmentation of neutrophil and eosinophil migration, neutrophil and monocyte aggregation, leukocyte adhesion, increased capillary permeability, and smooth muscle contraction. These effects contribute to inflammation, edema, mucus secretion, and bronchoconstriction in the airways of asthmatic patients. Zileuton relieves such symptoms through its selective inhibition of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Specifically, it inhibits leukotriene LTB4, LTC4, LTD4, and LTE4 formation. Both the R(+) and S(-) enantiomers are pharmacologically active as 5-lipoxygenase inhibitors in in vitro systems. Due to the role of leukotrienes in the pathogenesis of asthma, modulation of leukotriene formation by interruption of 5-lipoxygenase activity may reduce airway symptoms, decrease bronchial smooth muscle tone, and improve asthma control.






