


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
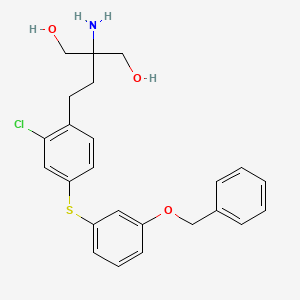

1. Mocravimod
2. Krp-203 Free Base
3. 509092-16-4
4. X71gcj0hli
5. 2-amino-2-(4-((3-(benzyloxy)phenyl)thio)-2-chlorophenethyl)propane-1,3-diol
6. 1,3-propanediol, 2-amino-2-(2-(2-chloro-4-((3-(phenylmethoxy)phenyl)thio)phenyl)ethyl)-
7. Unii-x71gcj0hli
8. Mocravimod [inn]
9. Mocravimod [who-dd]
10. Schembl641641
11. Gtpl9727
12. Krp203
13. Chembl2137148
14. Dtxsid30965173
15. Zinc6744940
16. Compound 2 [pmid: 23124563]
17. Ncgc00250388-01
18. Q27293620
19. 2-amino-2-[2-(4-{[3-(benzyloxy)phenyl]sulfanyl}-2-chlorophenyl)ethyl]propane-1,3-diol
20. 2-amino-2-[2-[2-chloro-4-(3-phenylmethoxyphenyl)sulfanylphenyl]ethyl]propane-1,3-diol
21. 2-amino-2-[2-[4-(3-benzyloxyphenylthio)-2-chlorophenyl]ethyl]-1,3-propanediol
| Molecular Weight | 444.0 g/mol |
|---|---|
| Molecular Formula | C24H26ClNO3S |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 443.1321926 g/mol |
| Monoisotopic Mass | 443.1321926 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


