



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0

1. D 18506
2. D-18506
3. D18506
4. Hdpc
5. Hexadecylphosphocholine
6. Impavido
7. Miltex
8. N-hexadecylphosphorylcholine
1. 58066-85-6
2. Hexadecylphosphocholine
3. Impavido
4. Miltex
5. Hdpc
6. Hexadecylphosphorylcholine
7. N-hexadecylphosphorylcholine
8. Miltefosinum
9. Miltefosina
10. 1-hexadecylphosphorylcholine
11. Hexadecyl 2-(trimethylammonio)ethyl Phosphate
12. Hexadecyl Phosphocholine
13. Miltefosin C
14. N-hexadecylphosphocholine
15. D-18506
16. Hexadecyl 2-(trimethylazaniumyl)ethyl Phosphate
17. Miltefosin
18. Nsc605583
19. Hexadecyl (2-(trimethylammonio)ethyl) Phosphate
20. Miltefosine (inn)
21. Monohexadecylphosphocholine
22. Chembl125
23. Nsc-605583
24. Nsc-758968
25. Monohexadecylphosphorylcholine
26. Hepc;hexadecyl Phosphocholine
27. 53ey29w7ec
28. Chebi:75283
29. Mmv688990
30. Ncgc00095169-01
31. Miltefos
32. Miltefosine [inn]
33. Dsstox_cid_25942
34. Dsstox_rid_81240
35. Dsstox_gsid_45942
36. Miltefosinum [inn-latin]
37. Miltefosina [inn-spanish]
38. C21h46no4p
39. Miltefosine [inn:ban]
40. Fos-choline 16
41. Cas-58066-85-6
42. D 18506
43. Brn 3690495
44. Unii-53ey29w7ec
45. Miltextrade Mark
46. Hepc Hydrate
47. Impavidotrade Mark
48. D18506
49. Impavido (tn)
50. Choline, Inner Salt
51. Mfcd00133396
52. Tf-002
53. 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-n,n,n-trimethylethanaminium Hydroxide, Inner Salt
54. Nsc 605583
55. Choline Hydroxide, Hexadecyl Hydrogen Phosphate, Inner Salt
56. Choline Phosphate, Hexadecyl Ester, Hydroxide, Inner Salt
57. Miltefosine [mi]
58. Hexadecyl Phosphorylcholine
59. H-1850
60. M-7200
61. Ethanaminium, 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-n,n,n-trimethyl-, Hydroxide, Inner Salt
62. Miltefosine [mart.]
63. Schembl26215
64. Miltefosine [who-dd]
65. 4-04-00-01460 (beilstein Handbook Reference)
66. Spectrum1505329
67. Dtxsid7045942
68. Gtpl11355
69. Miltefosine [orange Book]
70. Hexadecyl Phosphorylcholine Hydrate
71. Hms1922d16
72. Hms2089j15
73. Hms3649i09
74. Pharmakon1600-01505329
75. Hexadecylphosphocholine, Miltefosine
76. Bcp04506
77. Miltefosine (hexadecylphosphocholine)
78. Tox21_111466
79. Bdbm50034220
80. Ccg-35584
81. Ccg-36097
82. Ccg-40025
83. Dl-131
84. Hexadecyl 2-(trimethyl-.lambda.~5~-azanyl)ethyl Hydrogen Phosphate
85. Nsc758968
86. S3056
87. 1-n-hexadecylphosphorylcholine
88. Akos015914886
89. Tox21_111466_1
90. Bcp9000927
91. Db09031
92. Ncgc00095169-02
93. Ncgc00095169-03
94. Ncgc00095169-05
95. Ncgc00095169-07
96. Hy-13685
97. Bcp0726000071
98. Ft-0608148
99. M2445
100. Hexadecyloxy-2-trimethylammonioethylphosphorate
101. D02494
102. Ab00642217-03
103. Ab00642217_04
104. Miltefosine, >=98% (perchloric Acid Titration)
105. A831718
106. Q411787
107. Hexadecyl 2-(trimethylammonio)ethyl Phosphate Hydrate
108. 2-[hexadecoxy(hydroxy)phosphoryl]oxyethyl-trimethyl-ammonium
109. Phosphoric Acid Hexadecyl 2-(trimethylammonio)ethyl Ester
110. [2-(hexadecyloxy-hydroxy-phosphoryloxy)-ethyl]-trimethyl-ammonium
111. 3, 4-hydroxy-n,n,n-trimethyl-, Hydroxide, Inner Salt, 4-oxide
112. Hexadecyl 2-(trimethyl-lambda~5~-azanyl)ethyl Hydrogen Phosphate
113. Phosphoric Acid Hexadecyl 2-(trimethylammonio)ethyl Ester Hydrate
114. 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-n,n,n-trimethylethanaminium Hydroxide
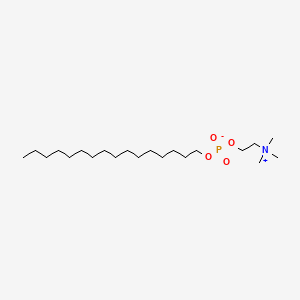
| Molecular Weight | 407.6 g/mol |
|---|---|
| Molecular Formula | C21H46NO4P |
| XLogP3 | 6.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 20 |
| Exact Mass | 407.31644595 g/mol |
| Monoisotopic Mass | 407.31644595 g/mol |
| Topological Polar Surface Area | 58.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 363 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Impavido |
| PubMed Health | miltefosine |
| Drug Classes | Anti-Infective Agent |
| Active Ingredient | Miltefosine |
| 2 of 2 | |
|---|---|
| Drug Name | Impavido |
| PubMed Health | miltefosine |
| Drug Classes | Anti-Infective Agent |
| Active Ingredient | Miltefosine |
For the treatment of mucosal (caused by Leishmania braziliensis), cutaneous (caused by L. braziliensis, L. guyanensis, and L. panamensis), and visceral leishmaniasis (caused by L. donovani). In comparing Leishmania drug susceptibility, it has been found that L. donovani is the most susceptible to miltefosine while L. major is the least susceptible. Off-label use includes treatment of free-living amebae (FLA) infections (unlabeled use; CDC, 2013).
FDA Label
Little is known about the clinical pharmacodynamics of miltefosine and other antileishmanial drugs.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CX - Other agents against leishmaniasis and trypanosomiasis
P01CX04 - Miltefosine
Absorption
After oral administration, miltefosine is slowly absorbed from the gastrointestinal tract with an absolute bioavailability of 82% in rats and 94% in dogs. Absolute bioavailability has not been assessed in humans, however GI absorption rate in a two-compartment model is estimated to be 0.416 hr-1.
Route of Elimination
Miltefosine is almost completely eliminated by degradation via phospholipase D. Drug keeps accumulating until the end of treatment due to the extremely slow elimination, as seen by the long elimination half lives.
Volume of Distribution
Radioactivity studies have found that miltefosine has a wide distribution with high levels in the kidney, intestinal mucosa, liver, and spleen.
Clearance
Plasma clearance is very low and the terminal elimination half life was found to be 84 and 159 hours in rats and dogs respectively.
Miltefosine is metabolized mainly by phospholipase D, releasing choline, choline-containing metabolites, and hexadecanol, which are likely to enter the intermediary metabolism. The metabolites produced by this reaction are all endogenous and are likely used for bio-synthesis of acetylcholine, cell membranes, and long-chain fatty acids.
The primary elimination half life is 7.05 days (range: 5.45-9.10 days) and the terminal half-life is 30.9 days (range: 30.8-31.2 days).
Miltefosine has demonstrated activity against Leishmania parasites and neoplastic cells primarily due to its effects on apoptosis and disturbance of lipid-dependent cell signalling pathways. Several potential antileishmanial mechanisms of action have been proposed, however no mechanism has been identified definitely. Within the mitochondria, miltefosine inhibits cytochrome-c oxidase leading to mitochondrial dysfunction and apoptosis-like cell death. Antineoplastic mechanisms of action are related to antileishmanial targets and include inhibition of phosphatidylcholine biosynthesis and inhibition of Akt (also known as protein kinase B), which is a crucial protein within the PI3K/Akt/mTOR intracellular signalling pathway involved in regulating the cell cycle. Animal studies also suggest it may be effective against Trypanosome cruzi (the organism responsible for Chagas' disease), metronidazole-resistant strains of Trichonomas vaginalis, and it may have broad-spectrum anti-fungal activity.






