
Synopsis
Synopsis
0
USDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
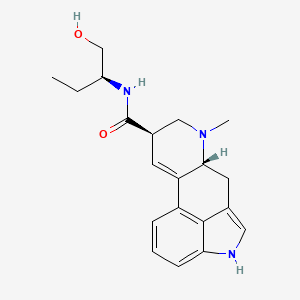

1. Mthergin
2. Methergin
3. Methergine
4. Methylergobasin
5. Methylergometrin
6. Methylergometrine
7. Methylergometrine Maleate
8. Methylergonovine Maleate
1. Methylergometrine
2. Methylergobasin
3. Methylergometrin
4. Methylergobasine
5. Methylergobrevin
6. Methylergonovin
7. 113-42-8
8. Methylergometrine Maleate
9. Ergotyl
10. Methergine
11. Methergen
12. Partergin
13. Basofortina
14. Methylergometrine (inn)
15. Ergometrine, Methyl-
16. W53l6fe61v
17. Chembl1201356
18. Me 277
19. D-lysergic Acid-(+)-butanolamide-(2)
20. (6ar,9r)-n-[(2s)-1-hydroxybutan-2-yl]-7-methyl-6,6a,8,9-tetrahydro-4h-indolo[4,3-fg]quinoline-9-carboxamide
21. Ncgc00017258-04
22. Metilergometrina
23. Metilergometrinio
24. Dsstox_cid_3283
25. Methylergometrinum
26. Metilergometrina [dcit]
27. Dsstox_rid_76957
28. Lysergic Acid Butanolamide
29. Dsstox_gsid_23283
30. Methylergometrine [inn]
31. Methylergometrine [inn:ban]
32. Lysergamide, N-((s)-1-(hydroxymethyl)propyl)-
33. Methylergometrinum [inn-latin]
34. Metilergometrinio [inn-spanish]
35. (8beta)-n-[(2s)-1-hydroxybutan-2-yl]-6-methyl-9,10-didehydroergoline-8-carboxamide
36. Ergotyl (tn)
37. D-lysergic Acid-dl-hydroxybutylamide-2
38. Hsdb 3364
39. N-(alpha-(hydroxymethyl)propyl)-d-lysergamide
40. Einecs 204-027-0
41. Unii-w53l6fe61v
42. Cas-113-42-8
43. H8d
44. 9,10-didehydro-n-(1-(hydroxymethyl)propyl)-6-methylergoline-8-carboxamide
45. 9,10-didehydro-n-(alpha-(hydroxymethyl)propyl)-6-methyl-ergoline-8-beta-carboxamide
46. Ergoline-8-carboxamide, 9,10-didehydro-n-(1-(hydroxymethyl)propyl)-6-methyl-, (8beta(s))-
47. Spectrum_000263
48. Prestwick3_000374
49. Spectrum2_000613
50. Spectrum3_000502
51. Spectrum5_001879
52. Biomol-nt_000154
53. Lopac0_000794
54. Schembl78176
55. Bspbio_000527
56. Bspbio_002023
57. Gtpl150
58. Kbioss_000743
59. Methylergonovine [mi]
60. Divk1c_000357
61. Spbio_000546
62. D-lysergic Acid 1-butanolamide
63. Bpbio1_000442
64. Bpbio1_000581
65. Methylergometrine [hsdb]
66. Methylergonovine [vandf]
67. Chebi:92607
68. Kbio1_000357
69. Kbio2_000743
70. Kbio2_003311
71. Kbio2_005879
72. Kbio3_001523
73. Dtxsid00904978
74. Ninds_000357
75. Methylergometrine [who-dd]
76. Tox21_110809
77. Bdbm50330860
78. Zinc95619105
79. Tox21_110809_1
80. Ccg-204878
81. Db00353
82. Sdccgsbi-0050771.p005
83. (8beta)-n-[(1s)-1-(hydroxymethyl)propyl]-6-methyl-9,10-didehydroergoline-8-carboxamide
84. Ergoline-8-beta-carboxamide, 9,10-didehydro-n-((s)-1-(hydroxymethyl)propyl)-6-methyl-
85. Idi1_000357
86. Ncgc00017258-03
87. Ncgc00017258-05
88. Ncgc00017258-06
89. Ncgc00017258-08
90. Ncgc00017258-12
91. Ncgc00024646-02
92. Ncgc00024646-03
93. Sbi-0050771.p004
94. Ab00514664
95. D08207
96. Ab00053497_03
97. Q424477
98. Brd-k34685430-001-01-1
99. Brd-k34685430-050-04-2
100. Brd-k34685430-050-06-7
101. (4r,7r)-n-[(2s)-1-hydroxybutan-2-yl]-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide
102. (8.beta.)-n-((1s)-1-(hydroxymethyl)propyl)-6-methyl-9,10-didehydroergoline-8-carboxamide
| Molecular Weight | 339.4 g/mol |
|---|---|
| Molecular Formula | C20H25N3O2 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 339.19467705 g/mol |
| Monoisotopic Mass | 339.19467705 g/mol |
| Topological Polar Surface Area | 68.4 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 549 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Methergine |
| PubMed Health | Methylergonovine (Injection) |
| Drug Classes | Uterine Stimulant |
| Drug Label | Methergine(methylergonovine maleate) is a semi-synthetic ergot alkaloid used for the prevention and controlof postpartum hemorrhage.Methergine is available in sterile ampuls of 1 mL, containing 0.2mg methylergonovine maleate for intramuscular... |
| Active Ingredient | Methylergonovine maleate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 0.2mg/ml; 0.2mg |
| Market Status | Prescription |
| Company | Edison Theraps |
| 2 of 2 | |
|---|---|
| Drug Name | Methergine |
| PubMed Health | Methylergonovine (Injection) |
| Drug Classes | Uterine Stimulant |
| Drug Label | Methergine(methylergonovine maleate) is a semi-synthetic ergot alkaloid used for the prevention and controlof postpartum hemorrhage.Methergine is available in sterile ampuls of 1 mL, containing 0.2mg methylergonovine maleate for intramuscular... |
| Active Ingredient | Methylergonovine maleate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 0.2mg/ml; 0.2mg |
| Market Status | Prescription |
| Company | Edison Theraps |
Oxytocics
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
For routine management after delivery of the placenta; postpartum atony and hemorrhage; subinvolution. Under full obstetric supervision, it may be given in the second stage of labor following delivery of the anterior shoulder. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Methylergonovine is a first-line agent for the treatment of postpartum hemorrhage; methylergonovine usually is given after oxytocin. Administration of parenteral ergot alkaloids during the third stage of labor decreases mean blood loss and the incidence of postpartum blood loss of 500 mL or more. /NOT included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
Ergonovine and methylergonovine should not be used for the induction or augmentation of labor.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3307
For more Therapeutic Uses (Complete) data for METHYLERGONOVINE (6 total), please visit the HSDB record page.
/Contraindications of methylergonovine therapy include:/ hypertension; toxemia; pregnancy; and hypersensitivity.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Use of Methergine is contraindicated during pregnancy because of its uterotonic effects.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
This drug should not be administered iv routinely because of the possibility of inducing sudden hypertensive and cerebrovascular accidents. If iv administration is considered essential as a lifesaving measure, methylergonovine should be given slowly over a period of no less than 60 seconds with careful monitoring of blood pressure. Intra-arterial or periarterial injection should be strictly avoided.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Caution should be exercised in the presence of sepsis, obliterative vascular disease, hepatic or renal involvement. Also use with caution during the second stage of labor. The necessity for manual removal of a retained placenta should occur only rarely with proper technique and adequate allowance of time for its spontaneous separation.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
For more Drug Warnings (Complete) data for METHYLERGONOVINE (13 total), please visit the HSDB record page.
For the prevention and control of excessive bleeding following vaginal childbirth
Methylergometrine is a semisynthetic ergot alkaloid and a derivative of ergonovine and is used for the prevention and control of postpartum and post-abortion hemorrhage. In general, the effects of all the ergot alkaloids appear to results from their actions as partial agonists or antagonists at adrenergic, dopaminergic, and tryptaminergic receptors. The spectrum of effects depends on the agent, dosage, species, tissue, and experimental or physiological conditions. All of the alkaloids of ergot significantly increase the motor activity of the uterus. After small doses contractions are increased in force or frequency, or both, but are followed by a normal degree of relaxation. As the dose is increased, contractions become more forceful and prolonged, resting tonus is markedly increased, and sustained contracture can result.
Oxytocics
Drugs that stimulate contraction of the myometrium. They are used to induce LABOR, OBSTETRIC at term, to prevent or control postpartum or postabortion hemorrhage, and to assess fetal status in high risk pregnancies. They may also be used alone or with other drugs to induce abortions (ABORTIFACIENTS). Oxytocics used clinically include the neurohypophyseal hormone OXYTOCIN and certain prostaglandins and ergot alkaloids. (From AMA Drug Evaluations, 1994, p1157) (See all compounds classified as Oxytocics.)
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02A - Uterotonics
G02AB - Ergot alkaloids
G02AB01 - Methylergometrine
Absorption
Absorption is rapid after oral (60% bioavailability) and intramuscular (78% bioavailability) administration.
Route of Elimination
Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Volume of Distribution
56.1 0 L
A delayed gastrointestinal absorption (Tmax about 3 hours) of methylergonovine tablets might be observed in postpartum women during continuous treatment with this oxytocic agent.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Pharmacokinetic studies following an iv injection have shown that methylergonovine is rapidly distributed from plasma to peripheral tissues within 2-3 minutes or less. The bioavailability after oral administration was reported to be about 60% with no accumulation after repeated doses. During delivery, with intramuscular injection, bioavailability increased to 78%. Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Bioavailability studies conducted in fasting healthy female volunteers have shown that oral absorption of a 0.2 mg methylergonovine tablet was fairly rapid with a mean peak plasma concentration of 3243 +/- 1308 pg/mL observed at 1.12 +/- 0.82 hours.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
For a 0.2 mg intramuscular injection, a mean peak plasma concentration of 5918 +/- 1952 pg/mL was observed at 0.41 +/- 0.21 hours. The extent of absorption of the tablet, based upon methylergonovine plasma concentrations, was found to be equivalent to that of the i.m. solution given orally, and the extent of oral absorption of the i.m. solution was proportional to the dose following administration of 0.1, 0.2, and 0.4 mg.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
For more Absorption, Distribution and Excretion (Complete) data for METHYLERGONOVINE (15 total), please visit the HSDB record page.
Hepatic, with extensive first-pass metabolism.
Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Drug Facts and Comparisons 2012. Wolters Kluwer Health St. Louis, MO 2012, p. 452
3.39 hours
The plasma level decline was biphasic with a mean elimination half-life of 3.39 hours (range 1.5 to 12.7 hours).
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Plasma concentrations of methylergonovine appear to decline in a biphasic manner. Following IV administration of methylergonovine to adults with normal renal function, the half-life of the drug in the initial phase (t1/2 alpha?) reportedly ranges from about 1-5 minutes and the half-life in the terminal phase (t1/2 beta) ranges from about 0.5-2 hours.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Methylergometrine acts directly on the smooth muscle of the uterus and increases the tone, rate, and amplitude of rhythmic contractions through binding and the resultant antagonism of the dopamine D1 receptor. Thus, it induces a rapid and sustained tetanic uterotonic effect which shortens the third stage of labor and reduces blood loss.
Methylergonovine acts directly on the smooth muscle of the uterus and increases the tone, rate, and amplitude of rhythmic contractions. Thus, it induces a rapid and sustained tetanic uterotonic effect which shortens the third stage of labor and reduces blood loss.
US Natl Inst Health; DailyMed. Current Medication Information for METHERGINE - methylergonovine maleate tablet, coated (February 2010). Available from, as of March 6, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b91d1729-2e8e-4398-9a0d-02763bcfe284
Ergonovine maleate and methylergonovine maleate are pharmacologically similar. Both drugs directly stimulate contractions of uterine and vascular smooth muscle. Following administration of usual therapeutic doses of ergonovine or methylergonovine, intense contractions of the uterus are produced and are usually followed by periods of relaxation. Larger doses of the drugs, however, produce sustained, forceful contractions followed by only short or no periods of relaxation. The drugs increase the amplitude and frequency of uterine contractions and uterine tone which in turn impede uterine blood flow. Ergonovine and methylergonovine also increase contractions of the cervix.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Ergonovine and methylergonovine produce vasoconstriction, mainly of capacitance vessels; increased central venous pressure, elevated blood pressure, and, rarely, peripheral ischemia and gangrene may result. Methylergonovine reportedly may interfere with prolactin secretion, but this effect has not been definitely established.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3308
Ergot alkaloids are antagonists of actions of 5-hydroxytryptamine and of certain metabolic actions of catecholamines. /Ergot alkaloids/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 876
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
69
PharmaCompass offers a list of Methylergonovine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methylergonovine manufacturer or Methylergonovine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methylergonovine manufacturer or Methylergonovine supplier.
PharmaCompass also assists you with knowing the Methylergonovine API Price utilized in the formulation of products. Methylergonovine API Price is not always fixed or binding as the Methylergonovine Price is obtained through a variety of data sources. The Methylergonovine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methylergonovine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methylergonovine, including repackagers and relabelers. The FDA regulates Methylergonovine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methylergonovine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methylergonovine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methylergonovine supplier is an individual or a company that provides Methylergonovine active pharmaceutical ingredient (API) or Methylergonovine finished formulations upon request. The Methylergonovine suppliers may include Methylergonovine API manufacturers, exporters, distributors and traders.
click here to find a list of Methylergonovine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Methylergonovine Drug Master File in Japan (Methylergonovine JDMF) empowers Methylergonovine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Methylergonovine JDMF during the approval evaluation for pharmaceutical products. At the time of Methylergonovine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Methylergonovine suppliers with JDMF on PharmaCompass.
A Methylergonovine CEP of the European Pharmacopoeia monograph is often referred to as a Methylergonovine Certificate of Suitability (COS). The purpose of a Methylergonovine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Methylergonovine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Methylergonovine to their clients by showing that a Methylergonovine CEP has been issued for it. The manufacturer submits a Methylergonovine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Methylergonovine CEP holder for the record. Additionally, the data presented in the Methylergonovine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Methylergonovine DMF.
A Methylergonovine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Methylergonovine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Methylergonovine suppliers with CEP (COS) on PharmaCompass.
Methylergonovine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methylergonovine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methylergonovine GMP manufacturer or Methylergonovine GMP API supplier for your needs.
A Methylergonovine CoA (Certificate of Analysis) is a formal document that attests to Methylergonovine's compliance with Methylergonovine specifications and serves as a tool for batch-level quality control.
Methylergonovine CoA mostly includes findings from lab analyses of a specific batch. For each Methylergonovine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methylergonovine may be tested according to a variety of international standards, such as European Pharmacopoeia (Methylergonovine EP), Methylergonovine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methylergonovine USP).

