
Synopsis
Synopsis
0
KDMF
0
NDC API
0
VMF
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
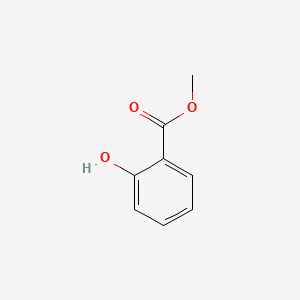

1. Hewedolor
2. Linsal
3. Methyl Salicylate Sodium Salt
4. Methylsalicylate
5. Metsal Liniment
6. Rheumabal
1. Methyl 2-hydroxybenzoate
2. 119-36-8
3. Wintergreen Oil
4. Gaultheria Oil
5. Betula Oil
6. Sweet Birch Oil
7. Teaberry Oil
8. Oil Of Wintergreen
9. Analgit
10. 2-hydroxybenzoic Acid Methyl Ester
11. Spicewood Oil
12. 2-carbomethoxyphenol
13. Gaultheriaoel
14. Wintergruenoel
15. Flucarmit
16. Betula
17. Exagien
18. Natural Wintergreen Oil
19. Methyl O-hydroxybenzoate
20. 2-(methoxycarbonyl)phenol
21. Methylsalicylate
22. Betula Lenta
23. Salicylic Acid, Methyl Ester
24. Benzoic Acid, 2-hydroxy-, Methyl Ester
25. Synthetic Wintergreen Oil
26. Gaultheria Oil, Artificial
27. Wintergreen Oil, Synthetic
28. Methyl2-hydroxybenzoate
29. O-hydroxybenzoic Acid, Methyl Ester
30. Fema No. 2745
31. Birch Oil, Sweet
32. Salicylic Acid Methyl Ester
33. Mfcd00002214
34. 68917-75-9
35. Flavor,wintergreen
36. Nsc 8204
37. 2-hydroxybenzoic Acid, Methyl Ester
38. Metylester Kyseliny Salicylove
39. Fema No. 2154
40. Fema No. 3113
41. Nsc-8204
42. 2-hydroxy-benzoic Acid Methyl Ester
43. Benzoic Acid, Hydroxy-, Methyl Ester
44. Methyl Salicylate,synthetic
45. Lav5u5022y
46. Chebi:31832
47. 135952-76-0
48. Ncgc00091106-02
49. Dsstox_cid_5659
50. Dsstox_rid_77872
51. Dsstox_gsid_25659
52. Caswell No. 577
53. Panalgesic
54. Theragesic
55. 2,4-cyclohexadien-1-one,6-(hydroxymethoxymethylene)-(9ci)
56. Predalure
57. Fema Number 2745
58. Black Birch Oil
59. Methyl Salicylate [jan]
60. Anthrapole Nd
61. Betula Lenta Oil
62. Ben Gay
63. Methyl Salicylate (natural)
64. 68917-50-0
65. Cas-119-36-8
66. Ccris 6259
67. Hsdb 1935
68. Winter Green Oil
69. Einecs 204-317-7
70. Epa Pesticide Chemical Code 076601
71. Metylester Kyseliny Salicylove [czech]
72. Methylester Kyseliny Salicylove [czech]
73. Brn 0971516
74. Unii-lav5u5022y
75. Wintergreen
76. Methyl Salicylate [jan:nf]
77. Methylester Kyseliny Salicylove
78. Ai3-00090
79. Methyl-salicylate
80. Salicylic Acid Methyl
81. 1-o-methylsalicylate
82. Salonpas (salt/mix)
83. Salicylate Methyl Ester
84. Birch Oil Sweet
85. Methyl Salicylate,(s)
86. Theragesic (salt/mix)
87. Methyl Salicylate (tn)
88. Methyl Salicylate, 8ci
89. Methyl-2-hydroxybenzoate
90. Enamine_001611
91. Teaberry Leaf Oil
92. Methyl Salicylate, 98%
93. Birch, Sweet, Oil
94. Methyl Salicylate, Bioxtra
95. Salicylic-acid Methyl Ester
96. Wln: Qr Bvo1
97. Ec 204-317-7
98. Wintergreen [vandf]
99. Wintergreen Leaf Oil
100. Schembl5312
101. Betula Oil, Wintergreen Oil
102. Checkerberry Leaf Oil
103. Sweet Birch Bark Oil
104. Betula Lenta Bark Oil
105. Cherry Birch Bark Oil
106. Mountain-tea Leaf Oil
107. Zinc490
108. 4-10-00-00143 (beilstein Handbook Reference)
109. 90045-28-6
110. Bidd:er0323
111. Wintergreen Oil [fcc]
112. Wintergreen Oil, China Origin
113. Methyl Salicylate [ii]
114. Methyl Salicylate [mi]
115. Wintergreen Oil [fhfi]
116. Chembl108545
117. Gtpl2431
118. Methyl Salicylate (jp17/nf)
119. Birch Sweet Oil [fhfi]
120. Methyl Salicylate [fcc]
121. Dtxsid5025659
122. Methyl Salicylate [fhfi]
123. Methyl Salicylate [hsdb]
124. Methyl Salicylate [inci]
125. Sweet Birch Oil [vandf]
126. Eastern Teaberry Leaf Oil
127. Fema 2745
128. Methyl Salicylate [vandf]
129. Nsc8204
130. Methyl Salicylate [mart.]
131. Hms1398j05
132. Hms2089h12
133. Hms3885c04
134. O-hydroxybenzoic Acid Methyl Ester
135. Methyl Salicylate [usp-rs]
136. Methyl Salicylate [who-dd]
137. Bcp29151
138. Cs-b1799
139. Hy-y0189
140. Methyl Ester 2-hydroxy-benzoic Acid
141. Tox21_111081
142. Tox21_201543
143. Tox21_300137
144. Bbl010504
145. S3756
146. Stk397388
147. Betula Lenta Bark Oil [inci]
148. Gaultheria Procumbens Leaf Oil
149. Methyl Salicylate, Analytical Standard
150. Akos000118977
151. Ccg-266225
152. Db09543
153. Methyl Ester Of 2-hydroxy-benzoic Acid
154. Methyl Salicylate [ep Impurity]
155. Methyl Salicylate [orange Book]
156. Methyl Salicylate [ep Monograph]
157. Methyl Salicylate, >=98%, Fcc, Fg
158. Ncgc00091106-01
159. Ncgc00091106-03
160. Ncgc00091106-04
161. Ncgc00091106-05
162. Ncgc00254104-01
163. Ncgc00259093-01
164. 8024-54-2
165. Ac-11584
166. Sy008800
167. Ts-02010
168. Methyl Salicylate,synthetic [vandf]
169. Salonpas Component Methyl Salicylate
170. Db-012808
171. Ft-0612582
172. Ft-0622968
173. Ft-0695782
174. Ft-0698844
175. Methyl Salicylate, Natural, 98%, Fcc, Fg
176. Methyl Salicylate Component Of Salonpas
177. Methyl Salicylate, Puriss., 99.0-100.5%
178. D01087
179. D70335
180. Methyl Salicylate, Saj First Grade, >=98.0%
181. Methyl Salicylate, Tested According To Ph.eur.
182. Ab01275470-01
183. Methyl Salicylate 100 Microg/ml In Acetonitrile
184. A804265
185. Methyl Salicylate, Reagentplus(r), >=99% (gc)
186. Methyl Salicylate, Vetec(tm) Reagent Grade, 99%
187. Q407669
188. Sr-05000001473
189. Gaultheria Procumbens (wintergreen) Leaf Oil
190. Q-100939
191. Sr-05000001473-1
192. Z19703590
193. 2-[hydroxy(methoxy)methylene]-3,5-cyclohexadiene-1-one
194. F0001-0306
195. 6-[(e)-methoxyhydroxymethylene]-2,4-cyclohexadiene-1-one
196. Gaultheria Procumbens (wintergreen) Leaf Oil [inci]
197. Methyl Salicylate, United States Pharmacopeia (usp) Reference Standard
198. Methyl Salicylate, Pharmaceutical Secondary Standard; Certified Reference Material
199. 9041-28-5
200. Gaultheria Oil Pound>>wintergreen Oil Pound>>2-hydroxy-benzoicacimethylester Pound>>methyl 2-hydroxybenzoate
| Molecular Weight | 152.15 g/mol |
|---|---|
| Molecular Formula | C8H8O3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 152.047344113 g/mol |
| Monoisotopic Mass | 152.047344113 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 144 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
OINTMENTS OR LINIMENTS CONTAINING METHYL SALICYLATE ARE APPLIED TOPICALLY AS COUNTERIRRITANTS FOR RELIEF OF PAIN ASSOCIATED WITH LUMBAGO, SCIATICA, AND RHEUMATIC CONDITIONS. FORMERLY USED INTERNALLY IN SMALL DOSES AS A CARMINATIVE.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 84:2404
MEDICATION (VET): ORALLY, PRIMARILY AS FLAVORING AGENT OR AS CARMINATIVE; TOPICALLY, AS IRRITANT OR COUNTERIRRITANT AIDED BY MASSAGE OR RUBBING AS IN UDDER OINTMENTS (1-3% CONCN), POULTICES & COUNTERIRRITANT MIXT (@ LEAST 5-10%) OVER SORE JOINT, MUSCLE, & BONE AREAS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 360
LOCAL ANALGESIC FOR HUMAN AND VETERINARY MEDICINE
SRI
OINTMENTS OR LINIMENTS ... . SHOULD NOT BE APPLIED TO BURNED AREAS OR TO OTHERWISE DAMAGED SKIN...USUALLY IN CONCN FROM 10-25% ... .
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 84:2404
ABSORPTION OF METHYL SALICYLATE CAN OCCUR THROUGH THE SKIN, & DEATH HAS RESULTED FROM SYSTEMIC POISONING FROM THE LOCAL MISAPPLICATION OF THE DRUG. IT IS A COMMON PEDIATRIC POISON, & ITS USE SHOULD BE STRONGLY DISCOURAGED.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 653
Children with fever and dehydration are particularly prone to intoxication from relatively small doses of salicylate. ... The use of aspirin is contraindicated in children and adolescents with febrile viral illnesses because of the risk of Reye's syndrome. /Salicylates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 651
4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG, BETWEEN 1 TEASPOON &1 OZ FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-205
Ointments or liniments containing methyl salicylate are applied topically as counter irritant for relief of acute pain associated with lumbago,sciatica and rheumatic conditions. Local analgesics for human and veterinary medicine.
Methyl salicylate relieve musculoskeletal pain in the muscles, joints, and tendons by causing irritation and reddening of the skin due to dilated capillaries and increased blood flow. It is pharmacologically similar to aspirin and other NSAIDs but as a topical agent it primarily acts as a rubefacient and skin irritant. Counter-irritation is believed to cause a soothing sensation of warmth.
Antirheumatic Agents
Drugs that are used to treat RHEUMATOID ARTHRITIS. (See all compounds classified as Antirheumatic Agents.)
Fixatives
Agents employed in the preparation of histologic or pathologic specimens for the purpose of maintaining the existing form and structure of all of the constituent elements. Great numbers of different agents are used; some are also decalcifying and hardening agents. They must quickly kill and coagulate living tissue. (See all compounds classified as Fixatives.)
Absorption
Approximately 12-20% of topically applied methyl salicylate may be systemically absorbed through intact skin within 10 hours of application, and absorption varies with different conditions such as surface area and pH. Dermal bioavailability is in the range of 11.8 30.7%. For the assessment of potential oral exposure to salicylates, bioavailability is assumed to be 100%.
Route of Elimination
Excreted by kidneys as free salicylic acid (10%), salicyluric acid (75%), salicylic phenolic (10%) and acyl glucuronide (5%), and gentisic acid (less than 1%).
Volume of Distribution
After absorption, methyl salicylate is distributed throughout most body tissues and most transcellular fluids, primarily by pH dependent passive processes. Salicylate is actively transported by a low-capacity, saturable system out of the CSF across the choroid plexus. The drug readily crosses the placental barrier.
MAY BE ABSORBED RAPIDLY THROUGH INTACT SKIN. BOWEL ABSORPTION IS SOMEWHAT ERRATIC ... ABSORBED AT LEAST IN PART AS THE INTACT ESTER AND SMALL AMT ARE EVEN EXCRETED AS SUCH BY THE KIDNEYS ... .
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-205
HUMAN SUBJECTS WERE GIVEN 7 MG/KG OF METHYL SALICYLATE BY MOUTH. AFTER 0.25 HOURS THE BLOOD CONCN WAS 1.28 MG%. AFTER 1.5 HOURS THE BLOOD CONCN WAS 1.33 MG%. /FROM TABLE/
Sunshine, I. (ed.). CRC Handbook of Analytical Toxicology. Cleveland: The Chemical Rubber Co., 1969., p. 362
At therapeutic doses, conjugation accounts for most salicylic elimination, whereas renal elimination becomes more important with large or multiple doses. A substantial first-pass effect occurs at therapeutic doses. /Salicylates/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 563
Orally ingested salicylates are absorbed rapidly, partly from the stomach but mostly from the upper small intestine. Appreciable conc are found in plasma in less than 30 min; after a single dose, a peak value is reached in about 2 hr and then gradually declines. /Salicylates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 649
For more Absorption, Distribution and Excretion (Complete) data for METHYL SALICYLATE (6 total), please visit the HSDB record page.
Minor metabolism may occur in various tissues but hepatic metabolism constitutes the majority of metabolic processes of absorbed methyl salicylate. It is mainly hydrolyzed to salicylic acid via hepatic esterase enzymes. Conjugation with glycine forms salicyluric acid and conjugation with glucuronic forms ester or acyl and ether or phenolic glucuronide, which are the three main metabolites.
...EVIDENCE THAT CONSIDERABLE HYDROLYSIS OF ESTER OCCURS IN INTESTINAL TRACT... IN SOME SPECIES, SUCH AS RABBIT, MAY BE PARTLY EXCRETED AS SULFATE OR GLUCURONIC ACID CONJUGATE ON THE FREE HYDROXYL GROUP. CONJUGATION APPEARS TO TAKE PLACE BEFORE HYDROLYSIS OF THE METHYL ESTER.
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1899
For small doses 80% of the hepatic metabolism results from conjugation with glycine to form salicyluric acid and with glucuronic acid to form salicyl acyl and phenolic glucuronide. The two parallel pathways (glycine, glucuronide conjugation) have limited capacity and saturate easily above therapeutic doses. /Salicylates/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 563
The biotransformation of salicylates takes place in many tissues, but particularly in the hepatic endoplasmic reticulum and mitochondria. The three chief metabolic products are salicyluric acid (the glycine conjugate), the ether or phenolic glucuronide, and the ester or acyl glucuronide. In addition, a small fraction is oxidized to gentisic acid (2,5-dihydroxybenzoic acid) and to 2,3-dihydroxybenzoic and 2,3,5-trihydroxybenzoic acids; gentisuric acid, the glycine conjugate of gentisic acid, is also formed. /Salicylates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 649
The plasma half-life for salicylate is 2 to 3 hr in low doses and about 12 hr at usual anti-inflammatory doses. The half-life of salicylate may be as long as 15 to 30 hr at high therapeutic doses or when there is intoxication.
The plasma half-life for ... salicylate is 2 to 3 hr in low doses and about 12 hr at usual antiinflammatory doses. The half-life of salicylate may be as long as 15 to 30 hr at high therapeutic doses or when there is intoxication. /Salicylates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 650
Counter-irritation is thought to be effective at alleviating musculoskeletal pain as the irritation of the sensory nerve endings is thought to alter or offset pain in the underlying muscle or joints that are served by the same nerves. This is thought to mask the underlying musculoskeletal pain and discomfort. When applied topically, methyl salicylate is thought to penetrate the skin and underlying tissues where it reversibly inhibits cyclooxygenase enzyme and locally and peripherally prevents the production of inflammatory mediators such as prostaglandin and thromboxane A2.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
49
PharmaCompass offers a list of Methyl Salicylate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methyl Salicylate manufacturer or Methyl Salicylate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methyl Salicylate manufacturer or Methyl Salicylate supplier.
PharmaCompass also assists you with knowing the Methyl Salicylate API Price utilized in the formulation of products. Methyl Salicylate API Price is not always fixed or binding as the Methyl Salicylate Price is obtained through a variety of data sources. The Methyl Salicylate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methyl Salicylate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methyl Salicylate, including repackagers and relabelers. The FDA regulates Methyl Salicylate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methyl Salicylate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methyl Salicylate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methyl Salicylate supplier is an individual or a company that provides Methyl Salicylate active pharmaceutical ingredient (API) or Methyl Salicylate finished formulations upon request. The Methyl Salicylate suppliers may include Methyl Salicylate API manufacturers, exporters, distributors and traders.
click here to find a list of Methyl Salicylate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Methyl Salicylate DMF (Drug Master File) is a document detailing the whole manufacturing process of Methyl Salicylate active pharmaceutical ingredient (API) in detail. Different forms of Methyl Salicylate DMFs exist exist since differing nations have different regulations, such as Methyl Salicylate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Methyl Salicylate DMF submitted to regulatory agencies in the US is known as a USDMF. Methyl Salicylate USDMF includes data on Methyl Salicylate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Methyl Salicylate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Methyl Salicylate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Methyl Salicylate Drug Master File in Japan (Methyl Salicylate JDMF) empowers Methyl Salicylate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Methyl Salicylate JDMF during the approval evaluation for pharmaceutical products. At the time of Methyl Salicylate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Methyl Salicylate suppliers with JDMF on PharmaCompass.
A Methyl Salicylate CEP of the European Pharmacopoeia monograph is often referred to as a Methyl Salicylate Certificate of Suitability (COS). The purpose of a Methyl Salicylate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Methyl Salicylate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Methyl Salicylate to their clients by showing that a Methyl Salicylate CEP has been issued for it. The manufacturer submits a Methyl Salicylate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Methyl Salicylate CEP holder for the record. Additionally, the data presented in the Methyl Salicylate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Methyl Salicylate DMF.
A Methyl Salicylate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Methyl Salicylate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Methyl Salicylate suppliers with CEP (COS) on PharmaCompass.
A Methyl Salicylate written confirmation (Methyl Salicylate WC) is an official document issued by a regulatory agency to a Methyl Salicylate manufacturer, verifying that the manufacturing facility of a Methyl Salicylate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Methyl Salicylate APIs or Methyl Salicylate finished pharmaceutical products to another nation, regulatory agencies frequently require a Methyl Salicylate WC (written confirmation) as part of the regulatory process.
click here to find a list of Methyl Salicylate suppliers with Written Confirmation (WC) on PharmaCompass.
Methyl Salicylate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methyl Salicylate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methyl Salicylate GMP manufacturer or Methyl Salicylate GMP API supplier for your needs.
A Methyl Salicylate CoA (Certificate of Analysis) is a formal document that attests to Methyl Salicylate's compliance with Methyl Salicylate specifications and serves as a tool for batch-level quality control.
Methyl Salicylate CoA mostly includes findings from lab analyses of a specific batch. For each Methyl Salicylate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methyl Salicylate may be tested according to a variety of international standards, such as European Pharmacopoeia (Methyl Salicylate EP), Methyl Salicylate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methyl Salicylate USP).

