
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
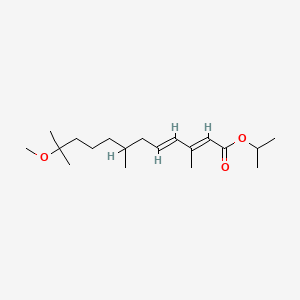

1. Altosid
2. Altosid Ps 10
3. Altosid Ps-10
4. Altosid Ps10
5. Zr 515
6. Zr-515
7. Zr515
1. 40596-69-8
2. Juvemon
3. Dianex
4. Kabat
5. Apex
6. Diacon
7. Pharoid
8. Precor
9. Manta
10. Minex
11. Methoprene S
12. Starbar Inhibitor
13. Manta (hormone)
14. Altosid Igr
15. Apex (pesticide)
16. Methoprene [inn]
17. Zr 515
18. Altosid
19. Altosid Sr 10
20. Altosid Liquid Larvicide
21. 36557-27-4
22. Propan-2-yl (2e,4e)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
23. Isopropyl 11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
24. Bioprene Bm Fire Ant Killer Bait
25. Oms 1697
26. Zpa 1019
27. Ent 70460
28. Isopropyl (2e,4e)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
29. (e,e)-1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
30. 2,4-dodecadienoic Acid, 11-methoxy-3,7,11-trimethyl-, 1-methylethyl Ester, (2e,4e)-
31. (e,e)-isopropyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
32. Isopropyl (2e,4e)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
33. Isopropyl (ee)-(rs)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
34. 2,4-dodecadienoic Acid, 11-methoxy-3,7,11-trimethyl-, 1-methylethyl Ester, (e,e)-
35. 8b830oj2ux
36. Chebi:34839
37. 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic Acid Isopropyl Ester
38. Zr-515
39. Methoprene (inn)
40. Ncgc00090777-05
41. Yuvemon
42. 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
43. 2,4-dodecadienoic Acid, 11-methoxy-3,7,11-trimethyl-, 1-methylethyl Ester
44. Isopropyl (2e,4e)-(7s)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
45. Dsstox_cid_12627
46. Dsstox_rid_79012
47. Dsstox_gsid_32627
48. Manina
49. Zodiac
50. Metoprag 20ce
51. Methoprenum
52. Metopreno
53. Caswell No. 028aaa
54. Methoprenum [inn-latin]
55. Metopreno [inn-spanish]
56. Dl-isopropyl 11-methoxy-3,7,11-trimethyl-trans-trans-2,4-dodecadienoate
57. (e,e)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic Acid 1-methylethyl Ester
58. Methoprene [iso]
59. Cas-40596-69-8
60. Hsdb 6926
61. Methoprene (unspecified)
62. Einecs 254-993-2
63. Isopropylmethoxytrimethyldodecadienoate
64. Epa Pesticide Chemical Code 105401
65. Unii-8b830oj2ux
66. Methoprene [ansi:bsi:iso]
67. Zpa-1019
68. Ent-70460
69. Sb-515
70. 52020-07-2
71. Ro 10-6425
72. Isopropyl-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
73. Methoprene [mi]
74. Methoprene [jan]
75. Dl-isopropyl 11-methoxy-3,7,11-trimethyl-trans-2,4-dodecadienoate
76. Isopropyl(2e,4e)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
77. Methoprene [hsdb]
78. 2,4-dodecadienoic Acid, 11-methoxy-3,7,11-trimethyl-, Isopropyl Ester
79. Methoprene [mart.]
80. Zodiac [veterinary] (tn)
81. Methoprene [who-dd]
82. Schembl19795
83. Mls001055477
84. Chembl291057
85. Dtxsid8032627
86. Chebi:39257
87. 2,4-dodecadienoic Acid, 11-methoxy-3,7,11-trimethyl-, 1-methylethyl Ester, (2z,4e,7s)-
88. Hms3039c11
89. Hy-b1161
90. Tox21_111019
91. Tox21_200654
92. Stk677200
93. Akos005594513
94. Isopropyl (2e,4e,7rs)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
95. Tox21_111019_1
96. Cs-4762
97. Methoprene 1000 Microg/ml In Acetone
98. Nsc 758655
99. (2e,4e)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic Acid 1-methylethyl Ester
100. Ncgc00090777-01
101. Ncgc00090777-02
102. Ncgc00090777-03
103. Ncgc00090777-04
104. Ncgc00090777-06
105. Ncgc00258208-01
106. Ncgc00263657-01
107. 41205-06-5
108. Smr001227198
109. Methoprene, Pestanal(r), Analytical Standard
110. D08200
111. Sr-01000854669
112. Q2329841
113. Sr-01000854669-2
114. Q27119793
115. Propan-2-yl 11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
116. (2e,4e)-isopropyl 11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
117. 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic Acid, 1-methylethyl Ester
118. (2e,4e)-3,7,11-trimethyl-11-methoxydodeca-2,4-dienoic Acid 1-methylethyl Ester
119. 1-methylethyl (2e,4e)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate
120. Isopropyl (e,e)-(rs)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate
| Molecular Weight | 310.5 g/mol |
|---|---|
| Molecular Formula | C19H34O3 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 11 |
| Exact Mass | 310.25079494 g/mol |
| Monoisotopic Mass | 310.25079494 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 378 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ The effects of methoprene, a juvenile hormone analogue (JHA), on Trypanosoma cruzi bloodstream trypomastigotes (Tulahuen strain, Tul 2 stock) were studied. It was observed that 150 uM of methoprene in in vitro experiments cause cellular death of T. cruzi. In contrast, methoprene was not able to clear bloodstream trypomastigotes in in vivo experiments, but it was observed a decrease of parasitemia levels of infected mice treated with 200 ug of methoprene/mouse/day during 5 days. According to these results and the low toxicity of methoprene, we suggest that this compound will serve as an effective agent to sterilize blood for transfusions.
PMID:12128051 Esteva M et al; Exp Parasitol 100 (4): 248-51 (2002)
/EXPL THER/ Drug therapy for the treatment of African sleeping sickness is limited by toxicity and resistance and in the last 50 years only one new drug has been introduced for the treatment of the human disease. We report that the juvenile hormone analog, methoprene, and several structurally related isoprenoid compounds kill Trypanosoma brucei in culture. Of the other isoprenoids tested, juvenile hormone III and mammalian retinoid X receptor ligands were the most potent trypanocides. Both the procyclic forms and the bloodstream trypomastigotes are killed by these compounds with LD50 values of 5-30 uM. Of the two methoprene stereoisomers, the EE form was the most active, suggesting that a protein target may be involved in mediating effects of these analogues against the parasite. Methoprene was not, however, able to clear trypanosomes from the blood of infected mice. Methoprene acid, the immediate downstream metabolite of methoprene, is not an effective anti-trypanosomal agent, suggesting that in the mice methoprene is converted to an inactive compound. Since methoprene and its analogues have low and well characterized toxicity in mammals these studies stress the importance of further exploring these isoprenoids as lead compounds for the treatment of African sleeping sickness.
PMID:9371088 Harmon MA et al; Exp Parasitol 87 (3): 229-236 (1997)
When (14)C methoprene was administered orally to rats, slightly less than 20% was excreted within 5 days in the urine and a similar amount in feces and almost 40% was excreted as (14)C02. About 17% was retained in the body. Highest concentrations were in liver (84.5 ppm), kidneys (29 ppm), lungs (26 ppm), fat (36.5 ppm), and the adrenal cortex (12-13 ppm). About 12 labeled compounds were detected in the urine but no unchanged methoprene was observed.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
Distribution and elimination of (14)C given to chickens as methoprene (14)C (isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienate 5 (14)C were investigated. When about 4 mg of methoprene was given in a single oral dose to colostomized chickens, elimination of (14)C was greatest in exhaled air; however, when 105 or 107 mg of methoprene was given, elimination of (14)C was greatest in urine. Up to 19% of the (14)C from a single dose of methoprene was eliminated over a 14 day period in the eggs of laying hens, and (14)C was detected in all tissues and organs examined.
Davison KL; J Agric Food Chem 24 (3): 641-3 (1976)
When the metabolic fate of methoprene (isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl- 2,4-dodecadienoate) was studied in a guinea pig, a steer, and a cow, a rather large percentage of the radiolabel was incorporated in the tissues and respired by the animals. In the urine and feces, a small amount of radiolabel was metabolized into free primary metabolites, somewhat more was incorporated into simple glucuronides, and a considerable quantity of radiolabel was found in polar compounds, possibly complex conjugates or polar biochemicals. No methoprene was found in the urine, but approximately 40% of the radiolabel in feces was contributed by unmetabolized methoprene. The formation of conjugates and the metabolism of methoprene was more extensive in the steer than in the guinea pig.
Chamberlain WF et al; J Agric Food Chem 23 (4): 736-42 (1975)
Treatment of Leghorn chickens with a single oral dose of (5-14C)methoprene (isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate) resulted in residual radioactivity in tissues and eggs. The chemical nature of the residual radiolabel in tissue (muscle, fat, liver), eggs, and excrement was thoroughly examined at several doses (0.6 to 77 mg/kg). Although a high initial dose (59 mg/kg) resulted in methoprene residues in muscle (0.01 ppm), fat (2.13 ppm), and egg yolk (8.03 ppm), these residues of methoprene represented only 39 and 2% of the total (14)C label in fat and egg yolk, respectively. Radiolabeled natural products from extensive degradation of methoprene were by far the most important 14C residues in tissues and eggs, particularly at the lower dose of 0.6 mg/kg where (14)C cholesterol and normal (14)C fatty acids (as triglyceride) contributed 8 and 71% of the total radiolabel in egg yolk. Novel minor metabolites of methoprene were observed in lipid depots, resulting from saturation of the dienoate system. These minor metabolites were conjugated to glycerol and/or cholesterol. radioactivity were found in the bile, liver, skin, fetus, and udder. In all species, approximately 40 percent of the radioactivity in the feces was due to unchanged methoprene. No methoprene was found in the urine.
Quistad GB et al; J Agric Food Chem 24 (3): 644-8 (1976)
For more Absorption, Distribution and Excretion (Complete) data for METHOPRENE (6 total), please visit the HSDB record page.
About 4 mg (14)C methoprene was administered orally to colostomized chickens. (14)C02 was the main (14)C product detected. When large doses were given, elimination was greatest in urine and (14)C was also found in the eggs and all tissues and organs examined ... . In addition to natural (14)C cholesterol and (14)C fatty acid triglycerides, there were metabolites conjugated to glycerol and/or cholesterol. Urine ... and ... feces contained compounds ... and each had undergone considerable isomerization. About 19% of the (14)C appeared in the eggs. Most of this was associated with egg proteins. The egg yolks also had radiolabeled fatty acid glycerides and cholesterol. Blood contained radiolabeled cholesterol and traces of cholesteryl esters. Tissue residues were similar to those found in eggs.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
A Hereford steer received a single oral dose of 5-(14)C-methoprene and sacrificed 2 weeks later. No primary metabolites were observed in fat, muscle, liver, lung, blood and bile. However, the majority of the tissue radioactivity was present as (14)C cholesterol. About 72% of the activity in bile appeared in cholesterol, cholic acid, and deoxycholic acid. Protein and cholesteryl esters of fatty acids also contained some radioactivity.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
When administered to a lactating cow, 5-(14)C-methoprene gave rise to randomly labeled acetate. This was incorporated into milk fat which was degraded to saturated and mono and di- enoic fatty acids. Labeled lactose, lactalbumin, casein, and free and esterified cholesterol was also observed ... . Similar qualitative results were observed in urine of a guinea pig orally dosed with methoprene. Quantitative differences were observed.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
Studies with housefly microsomal enzymes showed that the Beta-esterases present did not appreciably hydrolyze methoprene whereas other analogs were metabolized. Microsomal oxidase activity against juvenile hormone analogs was greater in resistant fly strains. ... Branched chain esters of methoprene analogs did not show significant difference in hydrolysis by housefly microsomal esterases. Methoprene was effective at 0.1 ug/pupa while others were ineffective at 10 ug/pupa.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
For more Metabolism/Metabolites (Complete) data for METHOPRENE (15 total), please visit the HSDB record page.
The degradation of methoprene by unidentified pond organisms was studied. The half-life in the pond water was about 30 h at 0.001 ppm and 40 h at 0.01 ppm.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
In wheat, the half-life of methoprene was estimated to be 3 to 7 weeks, depending on moisture content. The only metabolite observed was the free acid.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 333
Methoprene (isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate) is an insect juvenile hormone agonist that blocks metamorphosis in some insects. Recent evidence suggests that a metabolite, methoprene acid, activates vertebrate retinoid X receptors (RXRs), and may interfere with retinoic acid-regulated developmental processes. Methoprene, methoxy-methoprene acid, and two major breakdown products were tested for their ability to interfere with retinoid-regulated pathways when using transfected cells. The CV-1 cells were transiently transfected with genes encoding RXRs and response elements attached to luciferase reporters, and retinoic acid-sensitive F9 cells were stably transfected with retinoic acid receptor (RAR)/RXR response elements attached a lacZ reporter (Sil-REM/beta-gal-NEO). Experiments confirmed that methoxy-methopreneacid acted as a ligand for RXRs and was capable of activating transcription through RAR/RXR response elements. However, neither methoprenenor the breakdown products, 7-methoxycitronellal and 7-methoxycitronellic acid, activated transcription in transfected CV-1 or F9 cells.Methoprene and methoxy-methoprene acid may interfere with the conversion of all-trans-retinol and all-trans-retinaldehyde to all-trans-retinoic acid in the F9-derived cell line. Methoprene was as effective as the retinol dehydrogenase inhibitor citral in blocking the retinol-induced transcription of RAR/RXR-regulated reporter genes, whereas methoxy-methoprene acid blocked transcription stimulated by retinaldehyde.
PMID:15180384 Schoff PK, Ankley GT; Environ Toxicol Chem 23 (5): 1305-10 (2004)
In holometabolous insects such as mosquito, Aedes aegypti, midgut undergoes remodeling during metamorphosis. Insect metamorphosis is regulated by several hormones including juvenile hormone (JH) and 20-hydroxyecdysone (20E). The cellular and molecular events that occur during midgut remodeling were investigated by studying nuclear stained whole mounts and cross-sections of midguts and by monitoring the mRNA levels of genes involved in 20E action in methoprene-treated and untreated Ae. aegypti. We used JH analog, methoprene, to mimic JH action. In Ae. aegypti larvae, the programmed cell death (PCD) of larval midgut cells and the proliferation and differentiation of imaginal cells were initiated at about 36 hr after ecdysis to the 4th instar larval stage (AEFL) and were completed by 12 hr after ecdysis to the pupal stage (AEPS). In methoprene-treated larvae, the proliferation and differentiation of imaginal cells was initiated at 36h AEFL, but the PCD was initiated only after ecdysis to the pupal stage. However, the terminal events that occur for completion of PCD during pupal stage were blocked. As a result, the pupae developed from methoprene-treated larvae contained two midgut epithelial layers until they died during the pupal stage. Quantitative PCR analyses showed that methoprene affected midgut remodeling by modulating the expression of ecdysone receptor B, ultraspiracle A, broad complex, E93, ftz-f1, dronc and drice, the genes that are shown to play key roles in 20E action and PCD. Thus, JH analog, methoprene acts on Ae. aegypti by interfering with the expression of genes involved in 20E action resulting in a block in midgut remodeling and death during pupal stage.
PMID:16829058 Wu Y et al; Mech Dev 123 (7): 530-47 (2006)
... Here, a major malaria vector, Anopheles gambiae Giles, was used as a model insect to study the action of methoprene on female reproduction. Ecdysteroid titers and expression profiles of ecdysone-regulated genes were determined before and after a blood meal. An ecdysteroid peak was detected at 12 hr post blood meal (PBM). The maximum expression of ecdysone-regulated genes, such as ecdysone receptor (EcR), hormone receptor 3 (HR3) and vitellogenin (Vg) gene, coincided with the ecdysteroid peak. Interestingly, topical application of methoprene at 6 hr PBM delayed ovarian development and egg maturation by suppressing the expression of ecdysone-regulated genes in female mosquitoes. The data suggest that ecdysteroid titers are correlated with Vg synthesis, and methoprene affects vitellogenesis by modulating ecdysteroid action in A. gambiae.
PMID:20730984 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2928151 Bai H et al; Pest Manag Sci 66 (9): 936-43 (2010)
Insect growth regulator (juvenile hormone mimic), preventing metamorphosis to viable adults when applied to larval stages.
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 681

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
75
PharmaCompass offers a list of Methoprene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methoprene manufacturer or Methoprene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methoprene manufacturer or Methoprene supplier.
PharmaCompass also assists you with knowing the Methoprene API Price utilized in the formulation of products. Methoprene API Price is not always fixed or binding as the Methoprene Price is obtained through a variety of data sources. The Methoprene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methoprene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methoprene, including repackagers and relabelers. The FDA regulates Methoprene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methoprene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methoprene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methoprene supplier is an individual or a company that provides Methoprene active pharmaceutical ingredient (API) or Methoprene finished formulations upon request. The Methoprene suppliers may include Methoprene API manufacturers, exporters, distributors and traders.
click here to find a list of Methoprene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Methoprene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methoprene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methoprene GMP manufacturer or Methoprene GMP API supplier for your needs.
A Methoprene CoA (Certificate of Analysis) is a formal document that attests to Methoprene's compliance with Methoprene specifications and serves as a tool for batch-level quality control.
Methoprene CoA mostly includes findings from lab analyses of a specific batch. For each Methoprene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methoprene may be tested according to a variety of international standards, such as European Pharmacopoeia (Methoprene EP), Methoprene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methoprene USP).

