
Synopsis
Synopsis
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
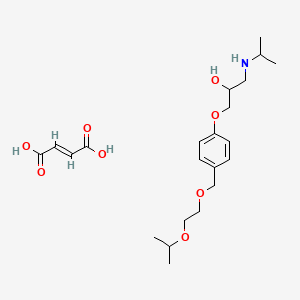

1. Bisoprolol
2. Bisoprolol Fumarate (1:1) Salt, (+-)-isomer
3. Bisoprolol Fumarate (2:1) Salt, (+-)-isomer
4. Bisoprolol Hydrochloride
5. Bisoprolol Methanesulfonate Salt
6. Bisoprolol, (+-)-isomer
7. Bisoprolol, (-)-isomer
8. Bisoprolol, Fumarate (1:1) Salt
9. Bisoprolol, Fumarate (2:1) Salt
10. Cl 297939
11. Cl-297939
12. Cl297939
13. Concor
14. Emd 33512
15. Emd-33512
16. Emd33512
17. Fumarate, Bisoprolol
18. Hydrochloride, Bisoprolol
1. 104344-23-2
2. Zebeta
3. Bisoprolol Hemifumarate
4. Bisoprolol Monofumarate
5. Fondril
6. Maintate
7. Concor
8. 105878-43-1
9. Bisoprolol (fumarate)
10. Bisoprolol Hemifumarate Salt
11. U057cx04h0
12. Emd33512
13. Ta-4708
14. Cl-297939
15. 1-[4-[[2-(1-methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol,(2e)-2-monobutenedioate
16. 2-propanol, 1-(4-((2-(1-methylethoxy)ethoxy)methyl)phenoxy)-3-((1-methylethyl)amino)-, (2e)-2-butenedioate (1:1)
17. 2-propanol, 1-(4-((2-(1-methylethoxy)ethoxy)methyl)phenoxy)-3-((1-methylethyl)amino)-, (2e)-2-butenedioate (1:1) (salt)
18. 2-propanol, 1-(4-((2-(1-methylethoxy)ethoxy)methyl)phenoxy)-3-((1-methylethyl)amino)-, (e)-2-butenedioate (1:1) (salt)
19. Smr000471619
20. Emd-33512
21. Bisocard
22. Cardicor
23. Congescor
24. Sr-01000597980
25. Bisoprololfumarate
26. Concor Cor
27. 66722-45-0
28. Bisoprolol Fumarate (1:x)
29. Schembl41419
30. Mls001076669
31. Mls001401436
32. Unii-u057cx04h0
33. Chembl1200708
34. Bcpp000331
35. Hms1569a21
36. Hms2051i16
37. Hms2094o19
38. Hms2233f12
39. Hms3884o05
40. Pharmakon1600-01505704
41. Nsc759181
42. S1206
43. Akos005267174
44. Ac-5016
45. Bcp9000419
46. Ccg-100883
47. Ccg-213520
48. Nc00133
49. Nsc-759181
50. Hy-120829
51. B3994
52. Cs-0079270
53. Sw197151-3
54. 104b232
55. Q-200729
56. Sr-01000597980-4
57. Q27290514
58. 1-(4-((2-isopropoxyethoxy)methyl)phenoxy)-3-(isopropylamino)propan-2-ol Hemi-fumarate
59. 1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)-2-propanol Fumaric Acid Salt
| Molecular Weight | 441.5 g/mol |
|---|---|
| Molecular Formula | C22H35NO8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 14 |
| Exact Mass | 441.23626707 g/mol |
| Monoisotopic Mass | 441.23626707 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 397 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Bisoprolol fumarate |
| Drug Label | Bisoprolol fumarate is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is ()-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-butenedioate... |
| Active Ingredient | Bisoprolol fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Teva Pharms; Unichem Pharms (usa); Aurobindo Pharma; Sandoz; Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Zebeta |
| PubMed Health | Bisoprolol (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | ZEBETA (bisoprolol fumarate) is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is ()-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-but... |
| Active Ingredient | Bisoprolol fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 3 of 4 | |
|---|---|
| Drug Name | Bisoprolol fumarate |
| Drug Label | Bisoprolol fumarate is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is ()-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-butenedioate... |
| Active Ingredient | Bisoprolol fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Teva Pharms; Unichem Pharms (usa); Aurobindo Pharma; Sandoz; Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Zebeta |
| PubMed Health | Bisoprolol (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | ZEBETA (bisoprolol fumarate) is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is ()-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-but... |
| Active Ingredient | Bisoprolol fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Teva Womens |
Adrenergic beta-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC BETA-1 RECEPTORS. (See all compounds classified as Adrenergic beta-1 Receptor Antagonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Sympatholytics
Drugs that inhibit the actions of the sympathetic nervous system by any mechanism. The most common of these are the ADRENERGIC ANTAGONISTS and drugs that deplete norepinephrine or reduce the release of transmitters from adrenergic postganglionic terminals (see ADRENERGIC AGENTS). Drugs that act in the central nervous system to reduce sympathetic activity (e.g., centrally acting alpha-2 adrenergic agonists, see ADRENERGIC ALPHA-AGONISTS) are included here. (See all compounds classified as Sympatholytics.)
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35650
Submission : 2021-02-16
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2015-09-11
Pay. Date : 2015-09-04
DMF Number : 15083
Submission : 2000-10-10
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-12-18
Pay. Date : 2013-08-02
DMF Number : 23241
Submission : 2009-11-02
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-03-01
Pay. Date : 2019-01-28
DMF Number : 18781
Submission : 2005-09-12
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19807
Submission : 2006-09-04
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2018-02-08
Pay. Date : 2017-10-10
DMF Number : 13910
Submission : 1998-12-22
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24120
Submission : 2010-12-09
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8024
Submission : 1989-04-12
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20120
Submission : 2006-12-11
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16398
Submission : 2003-01-29
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.
Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.
 Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.
Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.
Certificate Number : R1-CEP 2015-243 - Rev 00
Status : Valid
Issue Date : 2022-02-03
Type : Chemical
Substance Number : 1710
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Certificate Number : R0-CEP 2019-090 - Rev 00
Status : Valid
Issue Date : 2021-03-16
Type : Chemical
Substance Number : 1710
Certificate Number : CEP 2009-044 - Rev 02
Status : Valid
Issue Date : 2024-08-16
Type : Chemical
Substance Number : 1710
Certificate Number : R0-CEP 2020-095 - Rev 00
Status : Valid
Issue Date : 2022-05-04
Type : Chemical
Substance Number : 1710
Certificate Number : CEP 2016-139 - Rev 01
Status : Valid
Issue Date : 2024-01-23
Type : Chemical
Substance Number : 1710
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2007-336 - Rev 04
Status : Valid
Issue Date : 2023-12-13
Type : Chemical
Substance Number : 1710

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-143 - Rev 01
Status : Withdrawn by Holder
Issue Date : 2018-01-29
Type : Chemical
Substance Number : 1710

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2013-342 - Rev 02
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2019-05-13
Type : Chemical
Substance Number : 1710

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-287 - Rev 01
Status : Valid
Issue Date : 2023-04-24
Type : Chemical
Substance Number : 1710

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2015-127 - Rev 01
Status : Valid
Issue Date : 2024-03-05
Type : Chemical
Substance Number : 1710

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Registration Number : 218MF10893
Registrant's Address : Cesar Martinell i Brunet 12A, Poligono Rubi Sur, Rubi (Barcelona), Spain
Initial Date of Registration : 2006-11-10
Latest Date of Registration : 2019-09-11
Registration Number : 230MF10053
Registrant's Address : No. 8, Xiyuan Avenue, Hi-Tech District, Chengdu, 611731, China.
Initial Date of Registration : 2018-04-17
Latest Date of Registration : 2018-04-17
Registration Number : 301MF10029
Registrant's Address : Meissner Strasse 35 D-01445 Radebeul, Germany
Initial Date of Registration : 2019-07-05
Latest Date of Registration : 2019-07-05

Registration Number : 224MF10091
Registrant's Address : 326 Yokamachi, Toyama City, Toyama Prefecture
Initial Date of Registration : 2012-04-25
Latest Date of Registration : 2012-04-25

JP Bisoprolol Fumarate (for manufacturing purposes only)
Registration Number : 221MF10070
Registrant's Address : 326 Yokamachi, Toyama City, Toyama Prefecture
Initial Date of Registration : 2009-04-08
Latest Date of Registration : 2009-04-08

Registration Number : 220MF10126
Registrant's Address : Weisshausmatte, 6460 Altdorf UR, Switzerland
Initial Date of Registration : 2008-05-15
Latest Date of Registration : 2024-08-22

Registration Number : 217MF11012
Registrant's Address : WEISSHAUSMATTE CH-6460 ALTDORF SWITZERLAND
Initial Date of Registration : 2005-11-21
Latest Date of Registration : 2006-04-27

Registration Number : 217MF11134
Registrant's Address : 1-10-11 Nihonbashi Horidomecho, Chuo-ku, Tokyo
Initial Date of Registration : 2005-12-05
Latest Date of Registration : 2008-07-31

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Injectable / Parenteral, Suspension, Tablet
Grade : Parenteral, Oral, Topical
Category : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Application : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : Polysorbate 80 is used as a plasticizer, solubilizer, emulsifier, surfactant, and suspension stabilizer. It is also used in parenteral products.
Dosage Form : Tablet
Grade : Oral
Category : Fillers, Diluents & Binders, Film Formers & Plasticizers
Application : Fillers, Diluents & Binders, Film Formers & Plasticizers
Excipient Details : Copovidone is used as a tablet binder, a film-former and as part of the matrix material used in controlled-release formulations.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Polyvinylpyrrolidone/Vinyl Acetate Copolymer
Dosage Form : Tablet
Grade : Oral
Category : Controlled & Modified Release, Fillers, Diluents & Binders, Film Formers & Plasticizers, Solubilizers, Taste Masking
Application : Controlled & Modified Release, Fillers, Diluents & Binders, Film Formers & Plasticizers, Solubilizers, Taste Masking
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Polyvinylpyrrolidone/Vinyl Acetate Copolymer
Dosage Form : Capsule, Cream / Lotion / Ointment, Suspension, Tablet
Grade : Oral, Topical & Parenteral
Category : Solubilizers, Surfactant & Foaming Agents
Application : Solubilizers, Surfactant & Foaming Agents
Excipient Details : Polysorbate 80 acts as solubilizer, emulsifier and wetting agent.
Excipients Web Link
Grade : Oral
Category : Fillers, Diluents & Binders, Film Formers & Plasticizers
Application : Fillers, Diluents & Binders, Film Formers & Plasticizers
Excipient Details : Copovidone is used as a film-former and binding agent in oral solids such as tablets and capsules.
Pharmacopoeia Ref : N/A
Technical Specs : N/A
Ingredient(s) : Polyvinylpyrrolidone/Vinyl Acetate Copolymer
Dosage Form : Gel, Softgel Capsule, Solution, Suppository
Grade : Not Available
Category : Solubilizers
Application : Solubilizers
Excipient Details : Nonionic solubilizer, emulsifier and co-emulsifier
Dosage Form : Injectable / Parenteral, Tablet
Grade : Oral & Parenteral
Category : Emulsifying Agents, Solubilizers, Surfactant & Foaming Agents
Application : Emulsifying Agents, Solubilizers, Surfactant & Foaming Agents
Excipient Details : Polysorbate 80 is widely used as a surfactant, emulsifier, solubilizer, and stabilizer.
Brand Name : AFFINISOL HPMC HME
Application : Solubilizers
Excipient Details : Solubility enhancement, Spray-Dried Dispersion (SDD), Hot Melt Extrusion (HME)
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Paste, Shampoo, Solution, Syrup, Tablet
Grade : Topical, Oral
Category : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Excipient Details : Non-Ionic Hydrophilic Surfactant, Emulsifier, Solubilizer
Pharmacopoeia Ref : Ph.Eur, USP-NF
Technical Specs : HLB: 15, EO: 20; EXCiPACT
Ingredient(s) : Polysorbate 80
Brand Name : Polysorbate 80 Multi-Compendial
Application : Solubilizers
Excipient Details : A & C's Polysorbate 80 multi-compendial is an excipient which meets USP-NF, EP, BP and JP monographs.
Excipients Web Link
Global Sales Information

Main Therapeutic Indication : Cardiovascular
Currency : USD
2014 Revenue in Millions : -9%
2013 Revenue in Millions :
Growth (%) :

Main Therapeutic Indication : Cardiovascular Diseases
Currency : USD
2017 Revenue in Millions : 106
2016 Revenue in Millions : 116
Growth (%) : -9

Main Therapeutic Indication : Cardiovascular Diseases
Currency : USD
2018 Revenue in Millions : 50
2017 Revenue in Millions : 99
Growth (%) : -49%

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
08 Dec 2025
Reply
28 Jun 2025
Reply
20 May 2025
Reply
06 Sep 2024
Reply
04 Apr 2024
Reply
18 Jul 2023
Reply
10 Jun 2023
Reply
02 Feb 2023
Reply
29 Jul 2022
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
37
PharmaCompass offers a list of Bisoprolol Fumarate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bisoprolol Fumarate manufacturer or Bisoprolol Fumarate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bisoprolol Fumarate manufacturer or Bisoprolol Fumarate supplier.
PharmaCompass also assists you with knowing the Bisoprolol Fumarate API Price utilized in the formulation of products. Bisoprolol Fumarate API Price is not always fixed or binding as the Bisoprolol Fumarate Price is obtained through a variety of data sources. The Bisoprolol Fumarate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Merck Brand of Bisoprolol Fumarate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Merck Brand of Bisoprolol Fumarate, including repackagers and relabelers. The FDA regulates Merck Brand of Bisoprolol Fumarate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Merck Brand of Bisoprolol Fumarate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Merck Brand of Bisoprolol Fumarate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Merck Brand of Bisoprolol Fumarate supplier is an individual or a company that provides Merck Brand of Bisoprolol Fumarate active pharmaceutical ingredient (API) or Merck Brand of Bisoprolol Fumarate finished formulations upon request. The Merck Brand of Bisoprolol Fumarate suppliers may include Merck Brand of Bisoprolol Fumarate API manufacturers, exporters, distributors and traders.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Merck Brand of Bisoprolol Fumarate DMF (Drug Master File) is a document detailing the whole manufacturing process of Merck Brand of Bisoprolol Fumarate active pharmaceutical ingredient (API) in detail. Different forms of Merck Brand of Bisoprolol Fumarate DMFs exist exist since differing nations have different regulations, such as Merck Brand of Bisoprolol Fumarate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Merck Brand of Bisoprolol Fumarate DMF submitted to regulatory agencies in the US is known as a USDMF. Merck Brand of Bisoprolol Fumarate USDMF includes data on Merck Brand of Bisoprolol Fumarate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Merck Brand of Bisoprolol Fumarate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Merck Brand of Bisoprolol Fumarate Drug Master File in Japan (Merck Brand of Bisoprolol Fumarate JDMF) empowers Merck Brand of Bisoprolol Fumarate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Merck Brand of Bisoprolol Fumarate JDMF during the approval evaluation for pharmaceutical products. At the time of Merck Brand of Bisoprolol Fumarate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Merck Brand of Bisoprolol Fumarate Drug Master File in Korea (Merck Brand of Bisoprolol Fumarate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Merck Brand of Bisoprolol Fumarate. The MFDS reviews the Merck Brand of Bisoprolol Fumarate KDMF as part of the drug registration process and uses the information provided in the Merck Brand of Bisoprolol Fumarate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Merck Brand of Bisoprolol Fumarate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Merck Brand of Bisoprolol Fumarate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with KDMF on PharmaCompass.
A Merck Brand of Bisoprolol Fumarate CEP of the European Pharmacopoeia monograph is often referred to as a Merck Brand of Bisoprolol Fumarate Certificate of Suitability (COS). The purpose of a Merck Brand of Bisoprolol Fumarate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Merck Brand of Bisoprolol Fumarate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Merck Brand of Bisoprolol Fumarate to their clients by showing that a Merck Brand of Bisoprolol Fumarate CEP has been issued for it. The manufacturer submits a Merck Brand of Bisoprolol Fumarate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Merck Brand of Bisoprolol Fumarate CEP holder for the record. Additionally, the data presented in the Merck Brand of Bisoprolol Fumarate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Merck Brand of Bisoprolol Fumarate DMF.
A Merck Brand of Bisoprolol Fumarate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Merck Brand of Bisoprolol Fumarate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with CEP (COS) on PharmaCompass.
A Merck Brand of Bisoprolol Fumarate written confirmation (Merck Brand of Bisoprolol Fumarate WC) is an official document issued by a regulatory agency to a Merck Brand of Bisoprolol Fumarate manufacturer, verifying that the manufacturing facility of a Merck Brand of Bisoprolol Fumarate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Merck Brand of Bisoprolol Fumarate APIs or Merck Brand of Bisoprolol Fumarate finished pharmaceutical products to another nation, regulatory agencies frequently require a Merck Brand of Bisoprolol Fumarate WC (written confirmation) as part of the regulatory process.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Merck Brand of Bisoprolol Fumarate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Merck Brand of Bisoprolol Fumarate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Merck Brand of Bisoprolol Fumarate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Merck Brand of Bisoprolol Fumarate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Merck Brand of Bisoprolol Fumarate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Merck Brand of Bisoprolol Fumarate suppliers with NDC on PharmaCompass.
Merck Brand of Bisoprolol Fumarate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Merck Brand of Bisoprolol Fumarate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Merck Brand of Bisoprolol Fumarate GMP manufacturer or Merck Brand of Bisoprolol Fumarate GMP API supplier for your needs.
A Merck Brand of Bisoprolol Fumarate CoA (Certificate of Analysis) is a formal document that attests to Merck Brand of Bisoprolol Fumarate's compliance with Merck Brand of Bisoprolol Fumarate specifications and serves as a tool for batch-level quality control.
Merck Brand of Bisoprolol Fumarate CoA mostly includes findings from lab analyses of a specific batch. For each Merck Brand of Bisoprolol Fumarate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Merck Brand of Bisoprolol Fumarate may be tested according to a variety of international standards, such as European Pharmacopoeia (Merck Brand of Bisoprolol Fumarate EP), Merck Brand of Bisoprolol Fumarate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Merck Brand of Bisoprolol Fumarate USP).

