
Synopsis
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
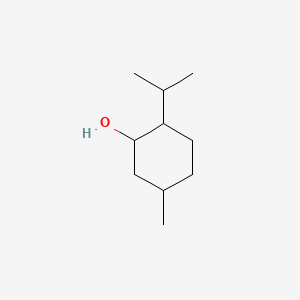

1. Cyclohexanol, 5-methyl-2-(1-methylethyl)-
2. Menthol, (1alpha,2beta,5alpha)-isomer
1. Dl-menthol
2. 1490-04-6
3. 2-isopropyl-5-methylcyclohexanol
4. 15356-70-4
5. Cyclohexanol, 5-methyl-2-(1-methylethyl)-
6. 89-78-1
7. P-menthan-3-ol
8. (+/-)-menthol
9. Hexahydrothymol
10. 5-methyl-2-propan-2-ylcyclohexan-1-ol
11. Menthol [usp]
12. Neomenthol
13. Rac-menthol
14. 5-methyl-2-(1-methylethyl)cyclohexanol
15. 5-methyl-2-(propan-2-yl)cyclohexan-1-ol
16. Chebi:25187
17. Menthyl Alcohol
18. (1r,2s,5r)-menthol
19. Menthol (usp)
20. Mfcd00001484
21. (1s, 2s, 5r)-(+)-neomenthol
22. 5-methyl-2-(propan-2-yl)cyclohexanol
23. Menthol 1000 Microg/ml In Acetonitrile
24. Dsstox_cid_805
25. Dsstox_rid_78794
26. Dsstox_gsid_29650
27. Racemic Menthol
28. Fema No. 2665
29. Caswell No. 540
30. 3-p-menthanol
31. Mentholum
32. Mineral Ice
33. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1s-(1.alpha.,2.alpha.,5.beta.)]-
34. Therapeutic Mineral Ice
35. 3-p-menthol
36. (+/-)-neoisomenthol
37. (+/-)-p-menthan-3-ol
38. Cas-1490-04-6
39. Ccris 9231
40. 3-hydroxy-p-menthane
41. Fisherman's Friend Lozenges
42. Robitussin Cough Drops
43. (1r,2r,5r)-isomenthol
44. Einecs 216-074-4
45. Epa Pesticide Chemical Code 051601
46. Menthol, Cis-1,3,trans-1,4-
47. 5-methyl-2-(1-methylethyl)-cyclohexanol
48. Ai3-08161
49. Hsdb 593
50. Menthol Racemate
51. Mfcd00062979
52. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)-
53. 2-isopropyl-5-methyl-cyclohexanol
54. (-)menthol
55. Ncgc00159382-02
56. (-) Menthol
57. 2-isopropyl-5-methylcyclohexan-1-ol
58. 4-isopropyl-1-methylcyclohexan-3-ol
59. Menthol, 99%
60. Dl-menthol (jp17)
61. 1-methyl-4-isopropyl-3-hydroxycyclohexane
62. Ec 216-074-4
63. Schembl4612
64. Menthol, (.+/-.)-
65. Menthol, Puriss., 99.0%
66. Chembl256087
67. Dl-menthol, Analytical Standard
68. Dtxsid8029650
69. Amy3077
70. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1s-(1.alpha.,2.beta.,5.beta.)]-
71. Fisherman's Friend Lozenges (tn)
72. Bdbm248162
73. Hms3744k19
74. 2-isopropyl-5-methylcyclohexanol #
75. Bcp27552
76. Bcp31841
77. Cs-m3737
78. Hy-n1369
79. Menthol Crystals; 15356-70-4
80. Tox21_200010
81. Tox21_303464
82. Bbl009325
83. Dl-menthol, >=95%, Fcc, Fg
84. Stk802468
85. ( Inverted Exclamation Marka)-menthol
86. Akos000119740
87. Akos016843634
88. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.alpha.,5.beta.)-
89. Am81446
90. Sb35230
91. Sb44308
92. Sb44857
93. (2r)-2-isopropyl-5-methyl-cyclohexanol
94. Menthol, Saj Special Grade, >=98.0%
95. Ncgc00159382-03
96. Ncgc00159382-04
97. Ncgc00159382-05
98. Ncgc00159382-06
99. Ncgc00257403-01
100. Ncgc00257564-01
101. Hy-75161
102. Sy004225
103. Sy010603
104. Vs-02042
105. Db-063989
106. Levomenthol; D-(-)-menthol; (-)-menthol
107. Cs-0016777
108. Ft-0600039
109. Ft-0604399
110. Ft-0604426
111. Ft-0604430
112. Ft-0620596
113. Ft-0625488
114. Ft-0695077
115. Ft-0695078
116. Ft-0695079
117. M0321
118. (+/-)-menthol, Racemic, >=98.0% (gc)
119. 2-isopropy-5-methylcyclohexanol-1,2,6,6-d4
120. D04849
121. D04918
122. E80543
123. (1s,2r,5r)-2-isopropyl-5-methyl-cyclohexanol
124. A808833
125. A809442
126. J-500418
127. Q27109870
128. Z1258992394
129. Menthol-plus It Inverted Exclamation Markas 3 Isomers-1,2,6,6-d4
130. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)-(.+/-.)-
131. Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1r-(1.alpha.,2.alpha.,5.beta.)]-
132. L-menthol; (-)-menthol; Levomenthol; Menthomenthol;2-isopropyl-5-methyl-cyclohexanol;menthol
133. Menthol Solution, Nmr Reference Standard, 30 Wt. % In Chloroform-d (99.8 Atom % D), Nmr Tube Size 5 Mm X 8 In.
134. Menthol Solution, Nmr Reference Standard, 50% In Chloroform-d (99.8 Atom % D), Chromium(iii) Acetylacetonate 0.5 %, Nmr Tube Size 5 Mm X 8 In.
| Molecular Weight | 156.26 g/mol |
|---|---|
| Molecular Formula | C10H20O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 156.151415257 g/mol |
| Monoisotopic Mass | 156.151415257 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipruritics
National Library of Medicine - Medical Subject Headings 2015 MeSH: Available from, as of June 2, 2015: https://www.nlm.nih.gov/cgi/mesh/2015/MB_cgi?term=menthol
EXPL THER Menthol has been tested in humans mainly for its pharmaceutical properties, such as enhancement of lung and airway volume.
OECD; Sreening Information Data Set (SIDS) Inital Assessment Report for SIDS Initial Assessment Meeting (SIAM) 16 Menthols(CASN 2216-51-5, 15356-60-2, 89-78-1, 1490-04-6) p. 9 (2003). Available from, as of June 2, 2015: https://www.inchem.org/pages/sids.html
Menthol, a natural product of the peppermint plant Mentha x piperita (Lamiaceae), is a monoterpene which is widely used as a natural product in cosmetics, a flavoring agent, and as an intermediate in the production of other compounds. Various extracts from peppermint contain menthol as a major active constituent and have been used for centuries as traditional medicines for a number of ailments including infections, insomnia, and irritable bowel syndrome as well as an insect repellent. /Traditional medicine//
PMID:23061635 Farco JA, Grundmann O; Mini Rev Med Chem. 13 (1): 124-31 (2013)
MEDICATION (VET): Its vapors have been used clinically in resp syndromes of horses, swine, & poultry. ... Parenterally, it is used in stimulant expectorant mixture...
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 334
For more Therapeutic Uses (Complete) data for MENTHOL (6 total), please visit the HSDB record page.
"Cooling" effect of l-menthol was found...to be superior to that produced by other isomers; odor and taste, too of l-menthol were superior, with some of the isomers producing sharp, irritating and disagreeable perceptions. /L-Menthol/
Osol, A., and R. Pratt. (eds.). The United States Dispensatory. 27th ed. Philadelphia: J.B. Lippincott, 1973., p. 697
Glucose-6-phosphate-dehydrogenase-deficiency in newborn babies may result in development of severe jaundice after menthol administration due to the inability of the neonates to conjugate menthol.
OECD; Sreening Information Data Set (SIDS) Inital Assessment Report for SIDS Initial Assessment Meeting (SIAM) 16 Menthols(CASN 2216-51-5, 15356-60-2, 89-78-1, 1490-04-6) p. 9 (2003). Available from, as of June 2, 2015: https://www.inchem.org/pages/sids.html
Sensitivity reactions associated with the use of mentholated products (including cigarettes) have been reported. Use of mentholated nasal drops in infants has evidently caused isolated cases of spasm of the larynx, and a few cases of nervous or digestive system disturbance have been associated with excessive inhalation or oral exposure to menthol.
BIBRA working group; Toxicity profile, The British Industrial Biological Research Association 7 (1936)
Menthol ... may cause allergic reactions (e.g. contact dermatitis, flushing, and headache) in certain individuals. Applying a menthol-containing ointment to the nostrils of infants for the treatment of cold symptoms may cause instant collapse.
Leung, A.Y., Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. New York, NY. John Wiley & Sons, Inc. 1996., p. 370
Vet: overdosing can cause convulsions &, eventually, death.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 334
4. 4= Very toxic: probable oral lethal dose (human) 50-500 mg/kg, between 1 teaspoon & 1 oz for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-258
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
...The percentage of a dose of l-menthol that is excreted combined with glucuronic acid in the rabbit depends on the magnitude of the dose; the larger the dose, the less is the conjugation. /L-Menthol/
Opdyke, D.L.J. (ed.). Monographs on Fragrance Raw Materials. New York: Pergamon Press, 1979., p. 519
Not all glucuronides are excreted by tubular secretion... conjugates of higher mol wt such as glucuronides of androsterone...are eliminated by glomerular filtration alone, whereas those of menthol...of lower mol wt...by tubules in addition to glomerular filtration... /DL-Menthol/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 158
Many substances with diverse structures are known to be excreted into bile; these incl glucuronides of menthol... /DL-Menthol/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 134
Absorption can occur from topical use. /DL-Menthol/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 334
For more Absorption, Distribution and Excretion (Complete) data for MENTHOL (7 total), please visit the HSDB record page.
In the United States, cigarette flavorings are banned, with the exception of menthol. The cooling effects of menthol could facilitate the absorption of tobacco toxicants. We examined levels of biomarkers of tobacco exposure among U.S. smokers of menthol and nonmenthol cigarettes. We studied 4,603 White, African-American, and Mexican-American current smokers 20 years of age or older who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 through 2010 and had data on cigarette type and serum cotinine, blood cadmium, and blood lead concentrations. Urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (NNAL) was studied in 1,607 participants with available measures. A total of 3,210 (74.3%) participants smoked nonmenthol cigarettes compared with 1,393 (25.7%) participants who smoked menthol cigarettes. The geometric mean concentrations comparing smokers of nonmenthol with menthol cigarettes were 163.1 versus 175.9 ng/mL for serum cotinine; 0.95 versus 1.02 ug/L for blood cadmium; 1.87 versus 1.75 ug/dL for blood lead; and 0.27 versus 0.23 ng/mL for urine NNAL. After multivariable adjustment, the ratios [95% confidence interval (CI)] comparing smokers of menthol with nonmenthol cigarettes were 1.03 (0.95-1.11) for cotinine, 1.10 (1.04-1.16) for cadmium, 0.95 (0.90-1.01) for lead, and 0.81 (0.65-1.01) for NNAL. In a representative sample of U.S. adult smokers, current menthol cigarette use was associated with increased concentration of blood cadmium, an established carcinogen and highly toxic metal, but not with other biomarkers. These findings provide information regarding possible differences in exposure to toxic constituents among menthol cigarette smokers compared with nonmenthol cigarette smokers./L-Menthol/
PMID:23250935 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3565051 Jones MR et al; Cancer Epidemiol Biomarkers Prev 22 (2): 224-32 (2013)
Researchers have recently suggested that nicotine and carcinogen exposure as measured by biomarkers such as cotinine and (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (NNAL) does not vary with cigarettes smoked per day (CPD) among Black smokers. Researchers have also suggested that nicotine exposure does not differ between menthol and nonmenthol smokers. In this study, we examine NNAL exposure for U.S. smokers by race, CPD, and menthol cigarette use. We analyzed urinary NNAL concentrations for more than 1500 everyday smokers participating in the National Health and Nutrition Examination Survey from 2007-2010. For purposes of comparison, we also analyzed serum cotinine concentrations for these smokers. We used linear regression analysis to estimate mean biomarker concentrations by CPD and race/ethnicity group and to examine the association between biomarker concentrations and menthol cigarette use by race/ethnicity group, controlling for other demographic and smoking characteristics. Biomarker concentrations increased with CPD for White, Black, and Hispanic smokers although NNAL concentrations leveled off for Black smokers at lower CPD levels compared with other smokers. Mean NNAL concentrations were lower among menthol smokers compared with nonmenthol smokers among smokers overall (beta = -0.165, p = .032) and White smokers (beta = -0.207, p = .048). We find evidence in national health survey data that nicotine and carcinogen exposure generally increases with CPD across race/ethnicity groups although the pattern of NNAL exposure differs by race/ethnicity group at high CPD levels. We also find evidence of differences in NNAL exposure for menthol smokers compared with nonmenthol smokers among smokers overall and White smokers /L-Menthol/
PMID:23089487 Rostron B; Nicotine Tob Res 15 (5): 950-6 (2013)
Corynebacterium sp. strain RWM1 grew with (-)-menthol, (-)-menthone and other acyclic monoterpenes as sole carbon sources. Growth on menthol was very slow, with a doubling time of more than 24 hr, and was not rapid with (-)-menthone (doubling time 12 hr). Concentrations of either carbon source greater than 0.025% inhibited growth. (-)-Menthone-grown cultures transiently accumulated 3,7-dimethyl-6-hydroxyoctanoate during growth, and (-)-menthol-grown cells oxidized (-)-menthol, (-)-menthone, 3,7-dimethyl-6-octanolide and 3,7-dimethyl-6-hydroxyoctanoate. Although neither a menthol oxidase nor a menthol dehydrogenase could be detected in extracts of (-)-menthol- or (-)-menthone-grown cells, an induced NADPH-linked monooxygenase with activity towards (-)-menthone was readily detected. With crude cell extracts, only 3,7-dimethyl-6-hydroxyoctanoate was detected as the reaction product. When the (-)-menthone monooxygenase was separated from an induced 3,7-dimethyl-6-octanolide hydrolase by chromatography on hydroxyapatite, the lactone 3,7-dimethyl-6-octanolide was shown to be the product of oxygenation. /L-Menthol/
Williams DR, PW Trudgill; Microbiology (Reading) 140 (3): 611-6 (1994)
L-Menthol was rapidly but incompletely glucuronidated. The output of l-menthol glucuronide was incr in all but 1 subject pretreated with cimetidine (1 g/day for 1 wk), an inhibitor of oxidative drug metabolism, & in all subjects pretreated with a drug-metabolizing enzyme inducer, phenobarbitone (60 mg nightly for 10 days). /L-Menthol/
BELL GD ET AL; BR J CLIN PHARMACOL 12 (2): 274 (1981)
For more Metabolism/Metabolites (Complete) data for MENTHOL (16 total), please visit the HSDB record page.
Exposure to low temperatures often causes allergic responses or urticaria. Similarly, menthol, a common food additive is also known to cause urticaria, asthma, and rhinitis. However, despite the obvious clinical implications, the molecular mechanisms responsible for inducing allergic responses to low temperatures and menthol have not been determined. Because a non-selective cation channel, transient receptor potential subtype M8 (TRPM8) is activated by cold and menthol, we hypothesized that this channel mediates cold- and menthol-induced histamine release in mast cells. Here, we report that TRPM8 is expressed in the basophilic leukemia mast cell line, RBL-2H3, and that exposure to menthol or low temperatures induced Ca(2+) influx in RBL-2H3 cells, which was reversed by a TRPM8 blocker. Furthermore, menthol, a TRPM8 agonist, induced the dose-dependent release of histamine from RBL-2H3 cells. When TRPM8 transcripts were reduced by siRNA (small interfering RNA), menthol- and cold-induced Ca(2+) influx and histamine release were significantly reduced. In addition, subcutaneous injection of menthol evoked scratching, a typical histamine-induced response which was reversed by a TRPM8 blocker. Thus, our findings indicate that TRPM8 mediates the menthol- and cold-induced allergic responses of mast cells, and suggest that TRPM8 antagonists be viewed as potential treatments for cold- and menthol-induced allergies. /DL-Menthol/
PMID:20934218 Cho Y et al; Cell Calcium. 48 (4): 202-8 (2010)
Menthol's characteristic cooling sensation is due, in part, to the activation of sensory neurons generally termed transient receptor potential (TRP) channels, in particular transient receptor potential melastatin family member 8 (TRPM8) and transient receptor potential subfamily A, member 1 (TRPA1). Menthol acts upon TRPM8 receptors by rapidly increasing intracellular calcium and mobilizing calcium flux through the channels to induce cold response signals at the application site. Aside from its cold-inducing sensation capabilities, menthol exhibits cytotoxic effects in cancer cells, induces reduction in malignant cell growth, and engages in synergistic excitation of GABA receptors and sodium ion channels resulting in analgesia. /DL-Menthol/
PMID:23061635 Farco JA, Grundmann O; Mini Rev Med Chem. 13 (1): 124-31 (2013)
In recent years, the transient receptor potential melastatin member 8 (TRPM8) channel has emerged as a promising prognostic marker and putative therapeutic target in prostate cancer. We have found that forced overexpression of TRPM8 in PC-3 cells can inhibit the cell proliferation and motility probably through the TRPM8 activation. In this study, we aimed to investigate whether activating the TRPM8 channel by its selective agonist menthol can inhibit the proliferation and motility of androgen-independent prostate cancer (AIPC) with remarkable expression of TRPM8. Menthol is a naturally occurring compound, which has been widely used in cosmetics and pharmaceutical products, and also as flavoring in food. DU145 cells are androgen-independent but have a remarkable expression of TRPM8. The demonstration of the existence of TRPM8 and the absence of TRPA1 in DU145 cells provided the foundation for the following experiments, because both TRPM8 and TRPA1 are molecular targets of menthol. The outcome of MTT assay indicated that menthol inhibited the cell growth (p < 0.01). Cell cycle distribution and scratch assay analysis revealed that menthol induced cell cycle arrest at the G(0)/G(1) phase (p < 0.01). Furthermore, menthol inhibited the migration of DU145 cells by downregulating the focal-adhesion kinase. So it suggests that the activation of the existing TRPM8 channels may serve as a potential and pragmatic treatment for those AIPC with remarkable expression of TRPM8, and menthol is a useful compound for future development as an anticancer agent. /DL-Menthol/
PMID:22437241 Wang Y et al; Pathol Oncol Res. 18 (4): 903-10 (2012)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
99
PharmaCompass offers a list of Menthol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Menthol manufacturer or Menthol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Menthol manufacturer or Menthol supplier.
PharmaCompass also assists you with knowing the Menthol API Price utilized in the formulation of products. Menthol API Price is not always fixed or binding as the Menthol Price is obtained through a variety of data sources. The Menthol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Menthol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Menthol, including repackagers and relabelers. The FDA regulates Menthol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Menthol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Menthol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Menthol supplier is an individual or a company that provides Menthol active pharmaceutical ingredient (API) or Menthol finished formulations upon request. The Menthol suppliers may include Menthol API manufacturers, exporters, distributors and traders.
click here to find a list of Menthol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Menthol DMF (Drug Master File) is a document detailing the whole manufacturing process of Menthol active pharmaceutical ingredient (API) in detail. Different forms of Menthol DMFs exist exist since differing nations have different regulations, such as Menthol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Menthol DMF submitted to regulatory agencies in the US is known as a USDMF. Menthol USDMF includes data on Menthol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Menthol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Menthol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Menthol Drug Master File in Japan (Menthol JDMF) empowers Menthol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Menthol JDMF during the approval evaluation for pharmaceutical products. At the time of Menthol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Menthol suppliers with JDMF on PharmaCompass.
A Menthol written confirmation (Menthol WC) is an official document issued by a regulatory agency to a Menthol manufacturer, verifying that the manufacturing facility of a Menthol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Menthol APIs or Menthol finished pharmaceutical products to another nation, regulatory agencies frequently require a Menthol WC (written confirmation) as part of the regulatory process.
click here to find a list of Menthol suppliers with Written Confirmation (WC) on PharmaCompass.
Menthol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Menthol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Menthol GMP manufacturer or Menthol GMP API supplier for your needs.
A Menthol CoA (Certificate of Analysis) is a formal document that attests to Menthol's compliance with Menthol specifications and serves as a tool for batch-level quality control.
Menthol CoA mostly includes findings from lab analyses of a specific batch. For each Menthol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Menthol may be tested according to a variety of international standards, such as European Pharmacopoeia (Menthol EP), Menthol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Menthol USP).

