
Synopsis
Synopsis
0
JDMF
0
FDA Orange Book
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 73-31-4
2. Melatonine
3. N-acetyl-5-methoxytryptamine
4. N-[2-(5-methoxy-1h-indol-3-yl)ethyl]acetamide
5. Circadin
6. 5-methoxy-n-acetyltryptamine
7. N-(2-(5-methoxy-1h-indol-3-yl)ethyl)acetamide
8. Melatol
9. Melovine
10. Acetamide, N-[2-(5-methoxy-1h-indol-3-yl)ethyl]-
11. N-[2-(5-methoxyindol-3-yl)ethyl]acetamide
12. N-(2-(5-methoxyindol-3-yl)ethyl)acetamide
13. N-acetyl-5-methoxy-tryptamine
14. Nsc 113928
15. Acetamide, N-(2-(5-methoxyindol-3-yl)ethyl)-
16. 8041-44-9
17. Acetamide, N-(2-(5-methoxy-1h-indol-3-yl)ethyl)-
18. Chembl45
19. Mfcd00005655
20. Nsc-56423
21. Jl5dk93rcl
22. Nsc-113928
23. 5-methoxy N-acetyl-tryptamine
24. 3-(n-acetyl-2-aminoethyl)-5-methoxyindole
25. Melatonex
26. Chebi:16796
27. Bci-049
28. Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]-
29. Melatonin (jan)
30. Nsc113928
31. J5.258b
32. Cas-73-31-4
33. Ncgc00015680-11
34. Melatonin [jan]
35. Dsstox_cid_2421
36. Dsstox_rid_76585
37. Dsstox_gsid_22421
38. Melapure
39. Posidorm
40. Primex
41. Wln: T56 Bmj D2mv1 Go1
42. [3h]melatonin
43. Melatonina (tn)
44. [3h]-melatonin
45. Ml1
46. Smr000326666
47. Ccris 3472
48. N-(2-(5-methoxyindol-3-yl)ethyl)-acetamide
49. N-[2-(5-methoxyindol-3-yl)ethyl]-acetamide
50. N-[2-(5-methoxy-1h-indol-3-yl)ethyl)acetamide
51. Sr-01000075559
52. Einecs 200-797-7
53. Unii-jl5dk93rcl
54. Brn 0205542
55. [3h]mlt
56. Melatonine;
57. Guna-dermo
58. Hsdb 7509
59. Melatobel (tn)
60. Tnp00300
61. Prestwick_312
62. N-[2-(5-methoxy-1h-indol-3-yl)-ethyl]-acetamide
63. Spectrum_000185
64. Melatonin [dsc]
65. Guna-dermo (salt/mix)
66. Melatonin [mi]
67. Primex (8ci,9ci)
68. Melatonin [hsdb]
69. Melatonin [inci]
70. Prestwick0_000458
71. Prestwick1_000458
72. Prestwick2_000458
73. Prestwick3_000458
74. Spectrum2_001344
75. Spectrum3_001393
76. Spectrum4_000066
77. Spectrum5_001745
78. Lopac-m-5250
79. Melatonin [vandf]
80. M1105
81. Chemdiv2_003916
82. Melatonin [mart.]
83. M 5250
84. M-1200
85. M-1250
86. Melatonin, >=99.5%
87. Melatonin [usp-rs]
88. Melatonin [who-dd]
89. Lopac0_000787
90. Oprea1_104553
91. Oprea1_814234
92. Schembl19018
93. Bspbio_000536
94. Bspbio_003006
95. Gtpl224
96. Kbiogr_000591
97. Kbioss_000665
98. Melatonin [ema Epar]
99. 5-22-12-00042 (beilstein Handbook Reference)
100. Acetamide, {n-[2-(5-methoxyindol-3-yl)ethyl]-}
101. Mls000859594
102. Mls001055382
103. Mls001240204
104. Bidd:er0618
105. Divk1c_000353
106. Spectrum1500690
107. Spbio_001527
108. Spbio_002475
109. Melatonin (synth.) Ultra-pure
110. Melatonin [green Book]
111. Bdbm9019
112. Bpbio1_000590
113. Gtpl1357
114. Acetamide, {n-[2-(5-methoxy-1h-indol-3-yl)ethyl]-}
115. Dtxsid1022421
116. Hms501b15
117. Kbio1_000353
118. Kbio2_000665
119. Kbio2_003233
120. Kbio2_005801
121. Kbio3_002226
122. Zinc57060
123. Melatonin 1.0 Mg/ml In Methanol
124. Ninds_000353
125. 3-n-acetyl-5-methoxyl Tryptamine
126. Glxc-25215
127. Hms1380b22
128. Hms1569k18
129. Hms1921e04
130. Hms2089f09
131. Hms2096k18
132. Hms2233d23
133. Hms3262m16
134. Hms3370j20
135. Hms3413p14
136. Hms3654a22
137. Hms3677p14
138. Hms3713k18
139. Hms3884m05
140. Melatonin (synth.) Standard-grade
141. Act03490
142. Amy33320
143. Bcp28154
144. Hy-b0075
145. Nsc56423
146. Tox21_110195
147. Tox21_201527
148. Tox21_302926
149. Tox21_500787
150. Ccg-38837
151. Hsci1_000400
152. Melatonin, Powder, >=98% (tlc)
153. Stk386880
154. Melatonin 100 Microg/ml In Methanol
155. Akos000276269
156. Tox21_110195_1
157. Cs-1769
158. Db01065
159. Ks-1454
160. Lp00787
161. Sdccgmls-0065812.p001
162. Sdccgmls-0065812.p002
163. Sdccgsbi-0050765.p003
164. Idi1_000353
165. Idi1_002631
166. Smp2_000309
167. N-acetyl-5-methoxy-tryptamine Melatonine
168. Ncgc00015680-01
169. Ncgc00015680-02
170. Ncgc00015680-03
171. Ncgc00015680-04
172. Ncgc00015680-05
173. Ncgc00015680-06
174. Ncgc00015680-07
175. Ncgc00015680-08
176. Ncgc00015680-09
177. Ncgc00015680-10
178. Ncgc00015680-12
179. Ncgc00015680-13
180. Ncgc00015680-14
181. Ncgc00015680-15
182. Ncgc00015680-16
183. Ncgc00015680-18
184. Ncgc00015680-35
185. Ncgc00090727-01
186. Ncgc00090727-02
187. Ncgc00090727-03
188. Ncgc00090727-04
189. Ncgc00090727-05
190. Ncgc00090727-06
191. Ncgc00090727-07
192. Ncgc00090727-08
193. Ncgc00090727-09
194. Ncgc00256404-01
195. Ncgc00259077-01
196. Ncgc00261472-01
197. Ac-10019
198. Ba164660
199. Nci60_004378
200. Sy051401
201. Ab00053279
202. Eu-0100787
203. Ft-0628191
204. Ft-0658928
205. Ft-0670984
206. S1204
207. Sw196607-4
208. C01598
209. D08170
210. Ab00053279-10
211. Ab00053279_12
212. {n-[2-(5-methoxyindol-3-yl)ethyl]-} Acetamide
213. 005m655
214. A929721
215. L001261
216. N-[2-(5-methoxy-1h-indol-3-yl)ethyl]-acetamide
217. Q180912
218. Sr-01000075559-1
219. Sr-01000075559-6
220. Sr-01000075559-7
221. Sr-01000075559-8
222. {n-[2-(5-methoxy-1h-indol-3-yl)ethyl]-} Acetamide
223. Brd-k97530723-001-07-6
224. Brd-k97530723-001-11-8
225. F1929-1777
226. Melatonin, British Pharmacopoeia (bp) Reference Standard
227. 0e2b08c1-b325-45b1-8939-6f9081efdfa4
228. Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]- (6ci,8ci)
229. Acetamide, N-[2-(5-methoxy-1h-indol-3-yl)ethyl]- (9ci)
230. Melatonin, United States Pharmacopeia (usp) Reference Standard
231. Melatonin, Pharmaceutical Secondary Standard; Certified Reference Material
232. Melatonin Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
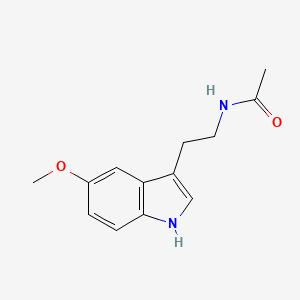
| Molecular Weight | 232.28 g/mol |
|---|---|
| Molecular Formula | C13H16N2O2 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 232.121177757 g/mol |
| Monoisotopic Mass | 232.121177757 g/mol |
| Topological Polar Surface Area | 54.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants, CNS Depressants
NLM Medical Subjects Headings; 2014 MeSH; Available from, as of January 29, 2014: https://www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?term=Melatonin
/In/ a crossover study involving seven totally blind subjects who had free-running circadian rhythms... the subjects were given 10 mg of melatonin or placebo daily, one hour before their preferred bedtime, for three to nine weeks. They were then given the other treatment. The timing of the production of endogenous melatonin was measured as a marker of the circadian time (phase), and sleep was monitored by polysomnography. At base line, the subjects had free-running circadian rhythms with distinct and predictable cycles averaging 24.5 hours (range, 24.2 to 24.9). These rhythms were unaffected by the administration of placebo. In six of the seven subjects the rhythm was entrained to a 24.0-hour cycle during melatonin treatment (P<0.001). After entrainment, the subjects spent less time awake after the initial onset of sleep (P=0.05) and the efficiency of sleep was higher (P=0.06). Three subjects subsequently participated in a trial in which a 10-mg dose of melatonin was given daily until entrainment was achieved. The dose was then reduced to 0.5 mg per day over a period of three months; the entrainment persisted, even at the lowest dose...
PMID:11027741 Sack RL et al; N Engl J Med 343 (15): 1070-7 (2000)
... Two probable physiologic effects have been associated with melatonin administration-the promotion of sleep onset and maintenance, and the phase-shifting of circadian rhythms, including the rhythm of melatonin itself. Both are produced by physiologic doses, i.e., 0.1-0.3 mg for sleep and 0.5 mg for phase-shifting. Melatonin's actions on sleep include both a direct action (which decreases sleep latency, increases sleep efficiency, and increases total sleep time) and an indirect effect on the daily rhythm in the phasing of sleep onset.
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)
A worldwide increase in the incidence of obesity indicates the unsuccessful battle against this disorder. Obesity and the associated health problems urgently require effective strategies of treatment. The new discovery that a substantial amount of functional brown adipose tissue (BAT) is retained in adult humans provides a potential target for treatment of human obesity. BAT is active metabolically and disposes of extra energy via generation of heat through uncoupling oxidative phosphorylation in mitochondria. The physiology of BAT is readily regulated by melatonin, which not only increases recruitment of brown adipocytes but also elevates their metabolic activity in mammals. It is speculated that the hypertrophic effect and functional activation of BAT induced by melatonin may likely apply to the human. Thus, melatonin, a naturally occurring substance with no reported toxicity, may serve as a novel approach for treatment of obesity. Conversely, because of the availability of artificial light sources, excessive light exposure after darkness onset in modern societies should be considered a potential contributory factor to human obesity as light at night dramatically reduces endogenous melatonin production. In the current article, the potential associations of melatonin, BAT, obesity and the medical implications are discussed.
PMID:20557470 Tan DX et al; Obes Rev 12 (3): 167-88. (2011)
For more Therapeutic Uses (Complete) data for MELATONIN (27 total), please visit the HSDB record page.
Doses <8 mg have reportedly induced heavy head, headache, and transient depression. May aggravate depression in patients with psychiatric illness... Some studies suggest melatonin may deepen depression in those who have it or induce it in those susceptible to it.
Duke, JA. Melatonin. In Handbook of Medicinal Herbs (2nd ed).p. 498. CRC Press, Boca Raton, FL (2002)
Melatonin in physiological doses causes vasoconstriction and also constricts cerebral arteries in rats.
Duke, JA. Melatonin. In Handbook of Medicinal Herbs (2nd ed).p. 498. CRC Press, Boca Raton, FL (2002)
Dose-related increases in plasma melatonin levels were observed, the 0.3 mg dose causing peak levels in the range usually observed nocturnally among young adults. When subjects received a higher dose (3.0 mg) but not 0.3 mg, plasma melatonin levels remained significantly elevated during much of the following day and the subjects exhibited hypothermia.
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)
While high doses of melatonin (10-450 mg/kg body weight parenterally) have sometimes elicited antioxidant effects in experimental animals in vivo, neither their long-term safety nor their effects on the animals' blood melatonin levels have been characterized. In humans- if not in nocturnally active lab rodents- such megadoses might ultimately impair sleep or various circadian rhythms, perhaps by downregulating melatonin receptors
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)
For more Drug Warnings (Complete) data for MELATONIN (10 total), please visit the HSDB record page.
Used orally for jet lag, insomnia, shift-work disorder, circadian rhythm disorders in the blind (evidence for efficacy), and benzodiazepine and nicotine withdrawal. Evidence indicates that melatonin is likely effective for treating circadian rhythm sleep disorders in blind children and adults. It has received FDA orphan drug status as an oral medication for this use. A number of studies have shown that melatonin may be effective for treating sleep-wake cycle disturbances in children and adolescents with mental retardation, autism, and other central nervous system disorders. It appears to decrease the time to fall asleep in children with developmental disabilities, such as cerebral palsy, autism, and mental retardation. It may also improve secondary insomnia associated with various sleep-wake cycle disturbances. Other possible uses for which there is some evidence for include: benzodiazepine withdrawal, cluster headache, delayed sleep phase syndrome (DSPS), primary insomnia, jet lag, nicotine withdrawal, preoperative anxiety and sedation, prostate cancer, solid tumors (when combined with IL-2 therapy in certain cancers), sunburn prevention (topical use), tardive dyskinesia, thrombocytopenia associated with cancer, chemotherapy and other disorders.
Slenyto is indicated for the treatment of insomnia in children and adolescents aged 2-18 with Autism Spectrum Disorder (ASD) and / or Smith-Magenis syndrome, where sleep hygiene measures have been insufficient.
Circadin is indicated as monotherapy for the short-term treatment of primary insomnia characterised by poor quality of sleep in patients who are aged 55 or over.
Treatment of insomnia
Primary insomnia
Melatonin is a hormone normally produced in the pineal gland and released into the blood. The essential amino acid L-tryptophan is a precursor in the synthesis of melatonin. It helps regulate sleep-wake cycles or the circadian rhythm. Production of melatonin is stimulated by darkness and inhibited by light. High levels of melatonin induce sleep and so consumption of the drug can be used to combat insomnia and jet lag. MT1 and MT2 receptors may be a target for the treatment of circadian and non circadian sleep disorders because of their differences in pharmacology and function within the SCN. SCN is responsible for maintaining the 24 hour cycle which regulates many different body functions ranging from sleep to immune functions
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Central Nervous System Depressants
A very loosely defined group of drugs that tend to reduce the activity of the central nervous system. The major groups included here are ethyl alcohol, anesthetics, hypnotics and sedatives, narcotics, and tranquilizing agents (antipsychotics and antianxiety agents). (See all compounds classified as Central Nervous System Depressants.)
N05CH01
N05CH01
N05CH01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CH - Melatonin receptor agonists
N05CH01 - Melatonin
Absorption
The absorption and bioavailability of melatonin varies widely.
To determine whether melatonin pharmacokinetics change during puberty, ... melatonin /was infused/ iv in 9 prepubertal, 8 pubertal, and 16 adult subjects and melatonin in serum and saliva and 6-hydroxymelatonin sulfate in urine /were measured/. A pilot study of 3 adult males showed dose linearity, absence of saturation kinetics, and unaltered metabolism and urinary excretion for doses of 0.1, 0.5, and 5.0 ug/kg. All other subjects received 0.5 ug/kg melatonin. The results of pharmacokinetic parameters calculated from serum melatonin showed no significant gender differences in adults. However, developmental differences were significant between prepubertal children and adults for terminal elimination rate constant (1.08 +/- 0.25 vs. 0.89 +/- 0.11 per hr), elimination half-life (0.67 +/- 0.12 vs. 0.79 +/- 0.10 hr), and area under the concentration-time curve (250.9 +/- 91.8 vs. 376.9 +/- 154.3 (pg/mL)hr, respectively). At all time points melatonin levels were higher in serum than in saliva, and the ratio between serum and salivary melatonin varied up to 55-fold within and between individuals. Results based on salivary melatonin showed significant differences between prepubertal children and adults for the terminal elimination rate constant (1.90 +/- 0.95 vs. 1.06 +/- 0.28 per hr). The described group differences in pharmacokinetic parameters suggest that prepubertal children metabolize melatonin faster than adults. The inconsistent ratio between serum and salivary melatonin calls for caution in the use of salivary melatonin for pharmacokinetic studies or to infer pineal function. The present findings, suggestive of faster melatonin metabolism in prepubertal children, combined with the known decline of serum melatonin with age and higher excretion rate of the metabolite in prepubertal children lead us to conclude that the prepubertal pineal gland has a higher melatonin secretion rate than the adult gland.
PMID:8626852 Cavallo A et al; J Clin Endocrinol Metab 81 (5): 1882-6 (1996)
The pharmacokinetics of melatonin during the day-time has been studied in 4 healthy subjects after a bolus i.v. injection of 5 or 10 ug/person and after a 5 hr infusion of 20 ug per person in 6 healthy subjects. In addition, a pinealomectomized patient whose nocturnal plasma melatonin had been abolished was investigated after the i.v. infusion--once during the night and once during the day. The clearance of melatonin from blood showed a biexponential decay. The pharmacokinetic parameters in the two studies were similar, except for the disappearance rate constant beta and the apparent volume of distribution at steady-state (Vss). Supplementary peaks or troughs were superimposed on the plateau and the falling part of the profile. They were not due to stimulation of endogenous secretion, because they were also seen in the pinealomectomized patient. During the melatonin infusion, the plasma hormone level reached a steady-state after 60 and 120 min, and when it was equal to the nocturnal level.
PMID:2340850 Mallo C et al; Eur J Clin Pharmacol 38 (3): 297-301 (1990)
... In a randomized and double-blind controlled study 10 healthy male subjects undertook an 80 min intensive hypertrophic heavy resistance exercise session (RES) for major muscles of the lower and upper extremities. The subjects were studied on two occasions receiving either melatonin (6 mg) or placebo (6 mg) in random order 60 min before each RES. Blood samples were taken from an antecubital vein both in fasting conditions in the morning and before RES (pre 60 min, pre 0 min), during RES (middle) and after RES (post 0 min, post 15 min, post 30 min, post 60 min). ... The serum melatonin concentration increased significantly (P<0.05-0.001) in the melatonin group following oral ingestion of melatonin and was elevated at every time point after that. The concentration reached a peak value of 1171.3+/-235.2 pg/mL in 60 min at pre 0. Serum melatonin increased slightly but significantly (P<0.05) also in the placebo group just before RES, in the middle of RES and after RES (post 0, post 15). There were large differences (P<0.01-0.001) in the serum melatonin concentration between the groups at all time points. ...
PMID:16506061 Mero AA et al; Eur J Appl Physiol 96 (6): 729-39 (2006)
...Pharmacokinetics of melatonin was studied in rats, dogs, and monkeys following intravenous and oral administrations, and the absolute oral bioavailability of melatonin was calculated from the area under the plasma concentration-time curve. The apparent elimination half-life of melatonin following an intravenous dose of 3 mg/kg (5 mg/kg in rats) was 19.8, 18.6, and 34.2 minutes, respectively, in rats, dogs, and monkeys. The dose normalized oral bioavailability of melatonin following a 10 mg/kg oral dose was 53.5% in rats, while it was in excess of 100% in dogs and monkeys. Further, bioavailability of melatonin following a 10 mg/kg intraperitoneal administration in rats was 74.0%, suggesting the lack of substantial first-pass hepatic extraction of melatonin in rats. However, the oral bioavailability of melatonin in dogs decreased to 16.9% following a 1 mg/kg oral dose, indicating dose-dependent bioavailability in dogs.
PMID:9062870 Yeleswaram K et al; J Pineal Res 22 (1): 45-51 (1997)
For more Absorption, Distribution and Excretion (Complete) data for MELATONIN (11 total), please visit the HSDB record page.
Hepatically metabolized to at least 14 identified metabolites (identified in mouse urine): 6-hydroxymelatonin glucuronide, 6-hydroxymelatonin sulfate, N-acetylserotonin glucuronide, N-acetylserotonin sulfate, 6-hydroxymelatonin, 2-oxomelatonin, 3-hydroxymelatonin, melatonin glucuronide, cyclic melatonin, cyclic N-acetylserotonin glucuronide, cyclic 6-hydroxymelatonin, 5-hydroxyindole-3-acetaldehyde, di-hydroxymelatonin and its glucuronide conjugate. 6-Hydroxymelatonin glucuronide is the major metabolite found in mouse urine (65-88% of total melatonin metabolites in urine).
Most of the melatonin in the circulation is inactivated in the liver where it is first oxidized to 6-hydroxy melatonin by a P450-dependent microsomal oxidase and then largely conjugated to sulfate or glucuronide before being excreted into urine or feces.
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)
In humans, the pineal hormone melatonin (MEL) is principally metabolized to 6-hydroxymelatonin (6-HMEL), which is further conjugated with sulfate and excreted in urine. MEL O-demethylation represents a minor reaction. The exact role of individual human cytochromes P450 (P450s) in these pathways has not been established. /The authors/ used a panel of 11 recombinant human P450 isozymes to investigate for the first time the 6-hydroxylation and O-demethylation of MEL. CYP1A1, CYP1A2, and CYP1B1 all 6-hydroxylated MEL, with CYP2C19 playing a minor role. These reactions were NADPH-dependent. CYP2C19 and, to some extent CYP1A2, O-demethylated MEL. The K(m) (uM) and V(max) (k(cat), pmol/ min/ pmol P450) for 6-hydroxylation were estimated as 19.2 +/- 2.01 and 6.46 +/- 0.22 (CYP1A1), 25.9 +/- 2.47 and 10.6 +/- 0.32 (CYP1A2), and 30.9 +/- 3.76 and 5.31 +/- 0.21 (CYP1B1). These findings confirm the suggestion of others that CYP1A2 is probably the foremost hepatic P450 in the 6-hydroxylation of MEL and a single report that CYP1A1 is also able to mediate this reaction. However, this is the first time that CYP1B1 has been shown to 6-hydroxylate MEL. The IC50 for the CYP1B1-selective inhibitor (E)-2,4,3',5'-tetramethoxystilbene was estimated to be 30 nM for MEL 6-hydroxylation by recombinant human CYP1B1. Comparison of brain homogenates from wild-type and cyp1b1-null mice revealed that MEL 6-hydroxylation was clearly mediated to a significant degree by CYP1B1. CYP1B1 is not expressed in the liver but has a ubiquitous extrahepatic distribution, and is found at high levels in tissues that also accumulate either MEL or 6-HMEL, such as intestine and cerebral cortex, where it may assist in regulating levels of MEL and 6-HMEL.
PMID:15616152 Ma X et al; Drug Metab Dispos 33 (4): 489-94 (2005)
Melatonin is synthesized at night in the human pineal gland and released into the blood and cerebrospinal fluid. It acts on the brains of humans to promote sleep, and also influences the phasing of sleep and various other circadian rhythms. During the day, plasma melatonin levels are low; at night, they rise 10 to 100-fold or more in young adults, but by considerably less in older people- who often may have frequent nocturnal awakenings as a consequence. Very small oral doses of melatonin raise daytime plasma melatonin to night-time levels, thus making it easier for people to fall asleep in the afternoon or evening. Such doses can also help older people remain asleep during the night. Melatonin has also occasionally been claimed to confer other medical benefits e.g. preventing such age-related diseases as atherosclerosis, cancer, and alzheimer's disease. The evidence in such claims is sparse.
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)
Melatonin has known human metabolites that include 6-Hydroxymelatonin, 6-[3-(2-Acetamidoethyl)-5-methoxyindol-1-yl]-3,4,5-trihydroxyoxane-2-carboxylic acid, and N-Acetyl-5-hydroxytryptamine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
35 to 50 minutes
...The terminal elimination rate constant (1.90 +/- 0.95 vs. 1.06 +/- 0.28 hr-1). ...
PMID:8626852 Cavallo A et al; J Clin Endocrinol Metab 81 (5): 1882-6 (1996)
Melatonin is a derivative of tryptophan. It binds to melatonin receptor type 1A, which then acts on adenylate cylcase and the inhibition of a cAMP signal transduction pathway. Melatonin not only inhibits adenylate cyclase, but it also activates phosphilpase C. This potentiates the release of arachidonate. By binding to melatonin receptors 1 and 2, the downstream signallling cascades have various effects in the body. The melatonin receptors are G protein-coupled receptors and are expressed in various tissues of the body. There are two subtypes of the receptor in humans, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2). Melatonin and melatonin receptor agonists, on market or in clinical trials, all bind to and activate both receptor types.The binding of the agonists to the receptors has been investigated for over two decades or since 1986. It is somewhat known, but still not fully understood. When melatonin receptor agonists bind to and activate their receptors it causes numerous physiological processes. MT1 receptors are expressed in many regions of the central nervous system (CNS): suprachiasmatic nucleus of the hypothalamus (SNC), hippocampus, substantia nigra, cerebellum, central dopaminergic pathways, ventral tegmental area and nucleus accumbens. MT1 is also expressed in the retina, ovary, testis, mammary gland, coronary circulation and aorta, gallbladder, liver, kidney, skin and the immune system. MT2 receptors are expressed mainly in the CNS, also in the lung, cardiac, coronary and aortic tissue, myometrium and granulosa cells, immune cells, duodenum and adipocytes. The binding of melatonin to melatonin receptors activates a few signaling pathways. MT1 receptor activation inhibits the adenylyl cyclase and its inhibition causes a rippling effect of non activation; starting with decreasing formation of cyclic adenosine monophosphate (cAMP), and then progressing to less protein kinase A (PKA) activity, which in turn hinders the phosphorilation of cAMP responsive element-binding protein (CREB binding protein) into P-CREB. MT1 receptors also activate phospholipase C (PLC), affect ion channels and regulate ion flux inside the cell. The binding of melatonin to MT2 receptors inhibits adenylyl cyclase which decreases the formation of cAMP.[4] As well it hinders guanylyl cyclase and therefore the forming of cyclic guanosine monophosphate (cGMP). Binding to MT2 receptors probably affects PLC which increases protein kinase C (PKC) activity. Activation of the receptor can lead to ion flux inside the cell.
Melatonin is implicated in numerous physiological processes, including circadian rhythms, stress, and reproduction, many of which are mediated by the hypothalamus and pituitary. The physiological actions of melatonin are mainly mediated by melatonin receptors. /The authors/ describe the distribution of the melatonin receptor MT1 in the human hypothalamus and pituitary by immunocytochemistry. MT1 immunoreactivity showed a widespread pattern in the hypothalamus. In addition to the area of the suprachiasmatic nucleus (SCN), a number of novel sites, including the paraventricular nucleus (PVN), periventricular nucleus, supraoptic nucleus (SON), sexually dimorphic nucleus, the diagonal band of Broca, the nucleus basalis of Meynert, infundibular nucleus, ventromedial and dorsomedial nucleus, tuberomamillary nucleus, mamillary body, and paraventricular thalamic nucleus were observed to have neuronal MT1 receptor expression. No staining was observed in the nucleus tuberalis lateralis and bed nucleus of the stria terminalis. The MT1 receptor was colocalized with some vasopressin (AVP) neurons in the SCN, colocalized with some parvocellular and magnocellular AVP and oxytocine (OXT) neurons in the PVN and SON, and colocalized with some parvocellular corticotropin-releasing hormone (CRH) neurons in the PVN. In the pituitary, strong MT1 expression was observed in the pars tuberalis, while a weak staining was found in the posterior and anterior pituitary. These findings provide a neurobiological basis for the participation of melatonin in the regulation of various hypothalamic and pituitary functions. The colocalization of MT1 and CRH suggests that melatonin might directly modulate the hypothalamus-pituitary-adrenal axis in the PVN, which may have implications for stress conditions such as depression.
PMID:17072839 Wu YH et al; J Comp Neurol 499 (6): 897-910 (2006)
A major mechanism through which melatonin reduces the development of breast cancer is based on its anti-estrogenic actions by interfering at different levels with the estrogen-signalling pathways. Melatonin inhibits both aromatase activity and expression in vitro (MCF-7 cells) as well as in vivo, thus behaving as a selective estrogen enzyme modulator. The objective of this study was to study the effect of MT1 melatonin receptor overexpression in MCF-7 breast cancer cells on the aromatase-suppressive effects of melatonin. Transfection of the MT1 melatonin receptor in MCF-7 cells significantly decreased aromatase activity of the cells and MT1-transfected cells showed a level of aromatase activity that was 50% of vector-transfected MCF-7 cells. The proliferation of estrogen-sensitive MCF-7 cells in an estradiol-free media but in the presence of testosterone (an indirect measure of aromatase activity) was strongly inhibited by melatonin in those cells overexpressing the MT1 receptor. This inhibitory effect of melatonin on cell growth was higher on MT1 transfected cells than in vector transfected ones. In MT1-transfected cells, aromatase activity (measured by the tritiated water release assay) was inhibited by melatonin (20% at 1 nM; 40% at 10 microM concentrations). The same concentrations of melatonin did not significantly influence the aromatase activity of vector-transfected cells. MT1 melatonin receptor transfection also induced a significant 55% inhibition of aromatase steady-state mRNA expression in comparison to vector-transfected MCF-7 cells (p<0.001). In addition, in MT1-transfected cells melatonin treatment inhibited aromatase mRNA expression and 1 nM melatonin induced a higher and significant down-regulation of aromatase mRNA expression (p<0.05) than in vector-transfected cells. The findings presented herein point to the importance of MT1 melatonin receptor in mediating the oncostatic action of melatonin in MCF-7 human breast cancer cells and confirm MT1 melatonin receptor as a major mediator in the melatonin signalling pathway in breast cancer.
PMID:17342341 Gonzalez A et al; Oncol Rep 17 (4): 947-53 (2007)
Almost all the melatonin formed in mammals is synthesized within the pineal gland... The tryptophan is first 5-hydroxylated (by the enzyme tryptophan hydroxylase) and then decarboxylated (by the enzyme aromatic L-amino acid decarboxylase) to form 5-hydroxytryptamine or serotonin. During daylight hours, the serotonin in pinealocytes tends to be stored, and is unavailable to enzymes (monoamine oxidase and the melatonin-forming enzymes) that would otherwise act on it. With the onset of darkness, postganglionic sympathetic outflow to the pineal increases, and the consequent release of norepinephrine onto pinealocytes causes stored serotonin to become accessible for intracellular metabolism. At the same time, the norepinephrine activates the enzymes (especially serotonin-N-acetyltransferase (SNAT), but also hydroxyindole-O-methyltransferase (HIOMT)) that convert serotonin to melatonin. Consequently, pineal melatonin levels rises manifold. ... The melatonin then diffuses out of the pineal gland into the blood stream and cerebrospinal fluid, rapidly raising human plasma melatonin levels from about 2-10 to 100-200 pg/mL.
Wurtman RJ; p. 457-464 in Encyclopedia of Dietary Supplements; Coates PM, Blackman MR, eds (2005)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
18
PharmaCompass offers a list of Melatonin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Melatonin manufacturer or Melatonin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Melatonin manufacturer or Melatonin supplier.
PharmaCompass also assists you with knowing the Melatonin API Price utilized in the formulation of products. Melatonin API Price is not always fixed or binding as the Melatonin Price is obtained through a variety of data sources. The Melatonin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Melatonin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Melatonin, including repackagers and relabelers. The FDA regulates Melatonin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Melatonin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Melatonin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Melatonin supplier is an individual or a company that provides Melatonin active pharmaceutical ingredient (API) or Melatonin finished formulations upon request. The Melatonin suppliers may include Melatonin API manufacturers, exporters, distributors and traders.
click here to find a list of Melatonin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Melatonin DMF (Drug Master File) is a document detailing the whole manufacturing process of Melatonin active pharmaceutical ingredient (API) in detail. Different forms of Melatonin DMFs exist exist since differing nations have different regulations, such as Melatonin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Melatonin DMF submitted to regulatory agencies in the US is known as a USDMF. Melatonin USDMF includes data on Melatonin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Melatonin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Melatonin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Melatonin Drug Master File in Korea (Melatonin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Melatonin. The MFDS reviews the Melatonin KDMF as part of the drug registration process and uses the information provided in the Melatonin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Melatonin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Melatonin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Melatonin suppliers with KDMF on PharmaCompass.
A Melatonin CEP of the European Pharmacopoeia monograph is often referred to as a Melatonin Certificate of Suitability (COS). The purpose of a Melatonin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Melatonin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Melatonin to their clients by showing that a Melatonin CEP has been issued for it. The manufacturer submits a Melatonin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Melatonin CEP holder for the record. Additionally, the data presented in the Melatonin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Melatonin DMF.
A Melatonin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Melatonin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Melatonin suppliers with CEP (COS) on PharmaCompass.
A Melatonin written confirmation (Melatonin WC) is an official document issued by a regulatory agency to a Melatonin manufacturer, verifying that the manufacturing facility of a Melatonin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Melatonin APIs or Melatonin finished pharmaceutical products to another nation, regulatory agencies frequently require a Melatonin WC (written confirmation) as part of the regulatory process.
click here to find a list of Melatonin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Melatonin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Melatonin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Melatonin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Melatonin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Melatonin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Melatonin suppliers with NDC on PharmaCompass.
Melatonin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Melatonin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Melatonin GMP manufacturer or Melatonin GMP API supplier for your needs.
A Melatonin CoA (Certificate of Analysis) is a formal document that attests to Melatonin's compliance with Melatonin specifications and serves as a tool for batch-level quality control.
Melatonin CoA mostly includes findings from lab analyses of a specific batch. For each Melatonin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Melatonin may be tested according to a variety of international standards, such as European Pharmacopoeia (Melatonin EP), Melatonin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Melatonin USP).

