



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
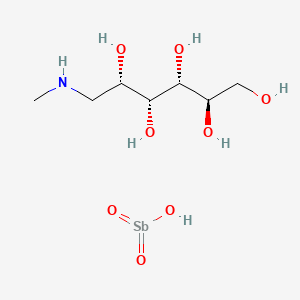

1. Meglumine Antimoniate
2. N Methylglucamine Antimonate
3. N-methylglucamine Antimonate
1. Meglumine Antimonate
2. Meglumine Antimoniate
3. N-methylglucamine Antimonate
4. 133-51-7
5. Methylglucamine Antimonate
6. Protostib
7. 75g4tw236w
8. N-methyl Glucamine Antimoniate
9. 2168-rp
10. Einecs 205-108-3
11. Glucantim
12. Unii-75g4tw236w
13. 1-deoxy-1-(methylamino)glucitol Antimonate(v)
14. 1-deoxy-1-(methylamino)-d-glucitol, Compound With Antimonic Acid (1:1)
15. D-glucitol, 1-deoxy-1-(methylamino)-, Compd. With Antimonic Acid (1:1)
16. Glucitol, 1-deoxy-1-(methylamino)-, Compd. With Antimonic Acid (1:1), D-
17. Schembl146372
18. Dtxsid4043935
19. D-glucitol, 1-deoxy-1-(methylamino)-, Trioxoantimonate(1-)
20. Mfcd01725422
21. Meglumine Antimonate [who-dd]
22. Akos025310684
23. Db13732
24. N-methylglucamine Antimonate [mi]
25. 133m517
26. Q2757969
27. (2r,3r,4r,5s)-6-(methylamino)hexane-1,2,3,4,5-pentaol Stibenate
28. D-glucitol, 1-deoxy-1-(methylamino)-, Compd. With Antimonic Acid (hsbo3) (1:1)
| Molecular Weight | 365.98 g/mol |
|---|---|
| Molecular Formula | C7H18NO8Sb |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 365.00705 g/mol |
| Monoisotopic Mass | 365.00705 g/mol |
| Topological Polar Surface Area | 168 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 180 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CB - Antimony compounds
P01CB01 - Meglumine antimonate


