Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
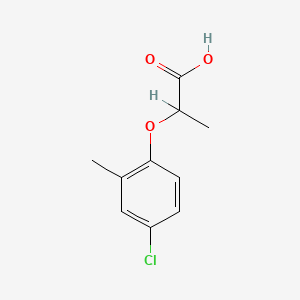

1. 2-(4-chloro-2-methylphenoxy)propionic Acid
2. 2-methyl-4-chlorophenoxypropionic Acid
3. Mecoprop, (+-)-isomer
4. Mecoprop, (r)-isomer
5. Mecoprop, (s)-isomer
6. Mecoprop, Ammonium Salt
7. Mecoprop, Potassium Salt
8. Mecoprop, Potassium Salt, (+-)-isomer
9. Mecoprop, Sodium Salt
10. Mecoprop, Sodium Salt, (+-)-isomer
1. 2-(4-chloro-2-methylphenoxy)propanoic Acid
2. 93-65-2
3. Compitox
4. Mecopeop
5. 2-(4-chloro-2-methylphenoxy)propionic Acid
6. 7085-19-0
7. Mechlorprop
8. Mecoturf
9. Rankotex
10. Mecprop
11. Morogal
12. Mepro
13. Anicon B
14. Isocarnox
15. Kilprop
16. Liranox
17. Mecopar
18. Mecoper
19. Mecopex
20. Runcatex
21. Proponex-plus
22. Cmpp
23. Iso-cornox
24. Okultin Mp
25. N.b. Mecoprop
26. Vi-par
27. Vi-pex
28. 2m4khp
29. Mecomec
30. 2-mcpp
31. 2-(2-methyl-4-chlorophenoxy)propionic Acid
32. Chipco Turf Herbicide Mcpp
33. Propanoic Acid, 2-(4-chloro-2-methylphenoxy)-
34. 2-(p-chloro-o-tolyloxy)propionic Acid
35. Fbc Cmpp
36. 2m-4cp
37. 2-(2-methyl-4-chlorophenoxy)propanoic Acid
38. 2m 4khp
39. Rd 4593
40. 2-(4-chlorophenoxy-2-methyl)propionic Acid
41. Propionic Acid, 2-(4-chloro-2-methylphenoxy)
42. Propionic Acid, 2-(2-methyl-4-chlorophenoxy)-
43. 2-(4-chloro-2-tolyloxy)propionic Acid
44. 2-(4-chloor-2-methyl-fenoxy)-propionzuur
45. 2-(4-chlor-2-methyl-phenoxy)-propionsaeure
46. 4-chloro-2-methylphenoxy-alpha-propionic Acid
47. Acido 2-(4-cloro-2-metil-fenossi)-propionico
48. Acide 2-(4-chloro-2-methyl-phenoxy)propionique
49. Kwas 4-chloro-2-metylofenoksypropionowy
50. (+)-alpha-(4-chloro-2-methylphenoxy) Propionic Acid
51. 2-(2-methyl-4-chlorphenoxy)-propionsaeure
52. Mls000084910
53. 74n8tkr9p8
54. Chembl272942
55. Chebi:75704
56. Propionic Acid, 2-[(4-chloro-o-tolyl)oxy]-
57. Kyselina 2-(4-chlor-2-methylfenoxy)propionova
58. .alpha.-(2-methyl-4-chlorophenoxy)propionic Acid
59. Celatox Cmpp
60. Nsc-60282
61. Mecoprop 10 Microg/ml In Acetonitrile
62. 2-(4-chloro-o-tolyloxy)propionic Acid
63. Mecoprop 100 Microg/ml In Acetonitrile
64. Smr000019256
65. Sys 67 Mecmin
66. Mecoturf;okultin Mp;2m4khp;nsc 60282
67. U 46 Kv Fluid
68. Dsstox_cid_4194
69. Propionic Acid, 2-((4-chloro-o-tolyl)oxy)-
70. Anicon P
71. 2-(2'-methyl-4'-chlorophenoxy)propionic Acid
72. Dsstox_rid_77323
73. Dsstox_gsid_24194
74. (+/-)-2-(4-chloro-2-methylphenoxy)propionic Acid
75. Caswell No. 559
76. Mecoprop [bsi:iso]
77. Cas-93-65-2
78. Mecoprop [iso]
79. Nsc 60282
80. Ccris 1464
81. (+/-)-mecoprop
82. Hsdb 1738
83. Einecs 202-264-4
84. Einecs 230-386-8
85. Epa Pesticide Chemical Code 031501
86. Brn 2212752
87. Unii-74n8tkr9p8
88. 2-(4-chloro-o-tolyl)oxylpropionic Acid
89. Compitox Plus
90. (rac)-mecoprop
91. Duplosan Cmpp
92. Alpha-(2-methyl-4-chlorophenoxy)propionic Acid
93. Astix Cmpp
94. Kwas 4-chloro-2-metylofenoksypropionowy [polish]
95. 2-(4-chloor-2-methyl-fenoxy)-propionzuur [dutch]
96. 2-(2-methyl-4-chlorphenoxy)-propionsaeure [german]
97. 2-(4-chlor-2-methyl-phenoxy)-propionsaeure [german]
98. Acido 2-(4-cloro-2-metil-fenossi)-propionico [italian]
99. Kyselina 2-(4-chlor-2-methylfenoxy)propionova [czech]
100. Iso-carnox
101. Acide 2-(4-chloro-2-methyl-phenoxy)propionique [french]
102. U 46 Kv-fluid
103. Opera_id_972
104. (+)-2-(4-chloro-2-methylphenoxy)propionic Acid
105. Mecoprop [mi]
106. Duplosan New System Cmpp
107. Ec 230-386-8
108. (+-)-2-((4-chloro-o-tolyl)oxy)propionic Acid
109. Schembl53198
110. (+/-)-2-(4-chloro-2-methylphenoxy)propanoic Acid
111. Wln: Qvy1&or Dg B1
112. Dtxsid9024194
113. Mecoprop, (+/-)-
114. Hms2234f04
115. Hms3371h17
116. Nsc60282
117. Tox21_201667
118. Tox21_303335
119. Bdbm50375464
120. Mfcd00002648
121. Pdsp1_001803
122. Pdsp2_001786
123. Stk208483
124. Akos000120873
125. Akos016890728
126. Rd-4593
127. O-(4-chloro-2-methylphenyl)lactic Acid
128. Ncgc00051613-02
129. Ncgc00051613-03
130. Ncgc00163831-01
131. Ncgc00257155-01
132. Ncgc00259216-01
133. (4-chloro-2-methylphenoxy)propionic Acid
134. Ts-03067
135. 4-chloro-2-methylphenoxy-a-propionic Acid
136. Am20041299
137. Ft-0608602
138. Ft-0670969
139. Ft-0704763
140. Ft-0770555
141. Mecoprop, Pestanal(r), Analytical Standard
142. 2-(4-chloro-o-tolyloxy)propionic Acid, 98%
143. C18742
144. 2-(2'-methyl-4'-chloro-phenoxy)-propionic Acid
145. 2-methyl-4-chlorophenoxy-.alpha.-propionic Acid
146. 4-chloro-2-methylphenoxy-.alpha.-propionic Acid
147. .alpha.-(4-chloro-2-methylphenoxy)propionic Acid
148. 085m190
149. Aj-087/41885651
150. L001008
151. Q149452
152. Sr-01000408149
153. Sr-01000408149-1
154. W-109051
155. (.+/-.)-2-(4-chloro-2-methylphenoxy)propanoic Acid
156. (.+/-.)-2-(4-chloro-2-methylphenoxy)propionic Acid
157. (+)-.alpha.-(4-chloro-2-methylphenoxy) Propionic Acid
158. 3,5-dibromo-2,4-dihydroxybenzoicacidmethylester
159. Propionic Acid, 2-[(4-chloro-o-tolyl)oxy]-, (.+/-.)-
160. Propanoic Acid, 2-(4-chloro-2-methylphenoxy)-, (.+/-.)-
| Molecular Weight | 214.64 g/mol |
|---|---|
| Molecular Formula | C10H11ClO3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 214.0396719 g/mol |
| Monoisotopic Mass | 214.0396719 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 208 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
Male and female Wistar rats with dosed orally with (14)C-Mecoprop-P (radiochemical purity: 99.5%, purity: 98.6%; spec. act.: 138.8 uCi/mg). Five rats/sex were dosed with 5 (Groups A, B, D) or 100 mg/kg (Groups C and E). The rats in Group B received 14 doses of unlabeled Mecoprop-P (purity: 99.8%) at 5 mg/kg/day prior to being dosed with the radiolabeled material. In addition,12 rats/sex were dosed with 5 mg/kg (Group F). In Groups A, B and C, urine and feces were collected for 7 days. Expired air was collected from two males in Group C. In Groups D and E, blood samples were collected for 7 days. In Group F, four animals/sex/timepoint were euthanized at 0.5, 3 and 6 hours after dosing. Absorption of the administered dose ranged from 82.92 to 100.47% with the absorption minimally reduced in the repeated dosing regimen and at the higher dosing level (males: A. 100.47%, B. 94.58%, C. 92.34%; females: A. 94.62%, B. 92.07%, C. 82.92%). Excretion was predominantly via the urine with the percentage of the total dose recovered in the urine and cage wash ranging from 79.74 to 100.06%. In Group A, 95.29 and 92.29% of the administered dose was collected in the urine and the cage wash within the first 24 hours for males and females, respectively. Repeated dosing reduced the amount collected in the urine and the cage wash during the first 24 hours to 88.97 and 86.46% for males and females, respectively. Likewise, at the 100 mg/kg dosing level, the percentage collected in the urine and the cage wash during the first 24 hours was reduced to 61.18 and 56.78% for males and females, respectively. The total radiolabel recovered in the feces ranged from 3.56 to 12.52% of the administered dose. No radiolabeled material was recovered in the expired air due to the positioning of the label on the phenyl ring. Radiolabel in the tissues and organs 7 days after dosing was predominantly in the fat followed by the skin, adrenals, kidneys and liver. Maximal levels of radioactivity were recovered from the various tissues and organs assayed within 3 hours of dosing (Group F). In the plasma pharmacokinetic analysis, Tmax values were 1.8 and 2.7 hours for males and females, respectively in Group D and 4.2 hours in Group E. /The half-life/ for elimination was 6.35 and 4.23 hours in Group D and 7.89 and 7.79 hours in Group E for males and females, respectively. ... /Mecoprop-p/
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, MCPP, Chemical Code Nos. 000374, 5333 p.9-10 (September 15, 1999). Available from, as of October 25, 2015: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Groups of 5 male Wistar rats were dosed orally with 5 mg/kg of either (14)C-Mecoprop-P-EHE (radiochemical purity: 99.6%, spec. act.: 145.37 uCi/mg) (Groups A and C) or (14)C-Mecoprop-P-DMA /(dimethylamine salt)/ (radiochemical purity (based on the acid): 99.8%, spec. act.: 114.79 uCi/mg) (Groups B and D). In Groups A and B (plasma pharmacokinetic study), blood samples were collected for 7 days. In Groups C and D, urine and feces were collected for 7 days. Expired air was collected for 48 hours. In the plasma pharmacokinetic analysis, Tmax values were 3.6 and 2 hr for Groups A and B, respectively. The half-life for elimination was 8.36 and 6.61 hours for Groups A and B, respectively. Absorption of the administered dose was at least 83.26 and 97.11% for Groups C and D, respectively (the total residual radiolabel in the tissues was not determined). Excretion was predominantly via the urine with the percentage of the total dose recovered in the urine 83.26 and 97.11%. In Groups C and D, respectively, 79.73 and 93.52% of the administered dose (calculated by the reviewer) was collected in the urine and the cage wash within the first 24 hours. The total radiolabel recovered in the feces was 3.29 and 4.68% of the administered dose for Groups C and D, respectively. No radiolabeled material was recovered in the expired air due to the positioning of the label on the phenyl ring. Radiolabel in the tissues and organs 7 days after dosing was largely limited to the skin and fat. The only metabolite identified in the study was hydroxymethyl-Mecoprop-P. Overall, unaltered test material and the hydroxylated metabolite constituted 96% of the recovered radiolabel in the first 48 hours after dosing. The parent material constituted 72.91 and 70.68% and the metabolite was 23.13 and 25.26% of the administered dose for Groups C and D, respectively.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, MCPP, Chemical Code Nos. 000374, 5333 p.8-9 (September 15, 1999). Available from, as of October 25, 2015: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
MCPP-p-DMAS (14)C-MCPP-p DMAS was incubated in vitro with rat plasma, stomach content, gastro-intestinal tract (GIT) or postmitochondrial liver fraction (S9) for 30 minutes. All incubated extracts were subjected to HPLC analysis. Results indicated that all of the administered (14)C-MCPP-p DMAS in plasma, stomach contents, gastro-intestinal tract and liver (S9) was present as the ionized form of (14)C-MCPP-p.
USEPA, Office of Prevention, Pesticides, and Toxic Substances; Revised HED Human Health Risk Assessment for Mecoprop (93-65-2) p.16 (July 2007). EPA Docket No.: EPA-HQ-OPP-2006-0943-0004. Available from, as of October 25, 2017: https://www.regulations.gov/
Male and female Wistar rats with dosed orally with (14)C-Mecoprop-P (radiochemical purity: 99.5%, purity: 98.6%; spec. act.: 138.8 uCi/mg). Five rats/sex were dosed with 5 (Groups A, B, D) or 100 mg/kg (Groups C and E). The rats in Group B received 14 doses of unlabeled Mecoprop-P (purity: 99.8%) at 5 mg/kg/day prior to being dosed with the radiolabeled material. In addition,12 rats/sex were dosed with 5 mg/kg (Group F). ... The only metabolite identified in the study was hydroxymethyl-Mecoprop-P. A greater percentage of this metabolite was recovered in the urine of the males. Overall, unaltered test material and the hydroxylated metabolite constituted 92.29 to 95.34% of the recovered radiolabel in the urine in the first 48 hours after dosing. For the males, the parent material was 52.17 to 67.08% and the metabolite was 28.26 to 41.39% of the administered dose. For the females these values ranged from 84.31 to 90.03% for the parent compound and 5.16 to 10.25% for the metabolite. /Mecoprop-p/
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, MCPP, Chemical Code Nos. 000374, 5333 p.9 (September 15, 1999). Available from, as of October 25, 2015: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
The dissociation of Mecoprop-p-DMA salt into Mecoprop-P acid and dimethyl amine was examined in various in vitro biological test systems. (14)C-Mecoprop-p-DMA was formed by mixing (14)C-Mecoprop-p acid (radiochemical purity: 99.5%, chemical purity: 98.6%, specific activity: 138.92 uCi/mg) with a dimethylamine solution. The test material was incubated with plasma (I), stomach contents (II), the gastrointestinal tract (III) and liver S9 fraction (IV) derived from male Wistar rats. The concentrations of the test material in the incubations were 0.1 (I), 5 (II), 0.35 (III) and 0.1 (IV) mg/mL. The samples were incubated for 30 minutes at 37o C. Study results indicated that the test material had largely dissociated into Mecoprop-P acid and DMA. It was not apparent from these results whether the dissociation may have been wholly or partially mediated enzymatically.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, MCPP, Chemical Code Nos. 000374, 5333 p.9 (September 15, 1999). Available from, as of October 25, 2015: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
In ...plants, degradation of the side-chain to 2-methyl-4-chlorophenol, ring hydroxylation and ring opening.
Hartley, D. and H. Kidd (eds.). The Agrochemicals Handbook. Old Woking, Surrey, United Kingdom: Royal Society of Chemistry/Unwin Brothers Ltd., 1983., p. A255/oct 83
Groups of 5 male Wistar rats were dosed orally with 5 mg/kg of either (14)C-Mecoprop-P-EHE (radiochemical purity: 99.6%, spec. act.: 145.37 uCi/mg) (Groups A and C) or (14)C-Mecoprop-P-DMA (radiochemical purity (based on the acid): 99.8%, spec. act.: 114.79 uCi/mg) (Groups B and D). ... The half-life for elimination was 8.36 and 6.61 hours for Groups A and B, respectively. ...
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, MCPP, Chemical Code Nos. 000374, 5333 p.8 (September 15, 1999). Available from, as of October 25, 2015: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
/In/ two cases of serious intoxication with phenoxy herbicides (MCPP) ... The plasma half-life was about 17 hr. MCPP plasma elimination probably follows first-order kinetics.
PMID:3391629 Meulenbelt J et al; Hum Toxicol 7 (3): 289-92 (1988)
ABOUT THIS PAGE
47
PharmaCompass offers a list of Mecoprop API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mecoprop manufacturer or Mecoprop supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mecoprop manufacturer or Mecoprop supplier.
PharmaCompass also assists you with knowing the Mecoprop API Price utilized in the formulation of products. Mecoprop API Price is not always fixed or binding as the Mecoprop Price is obtained through a variety of data sources. The Mecoprop Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mecoprop manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mecoprop, including repackagers and relabelers. The FDA regulates Mecoprop manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mecoprop API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Mecoprop supplier is an individual or a company that provides Mecoprop active pharmaceutical ingredient (API) or Mecoprop finished formulations upon request. The Mecoprop suppliers may include Mecoprop API manufacturers, exporters, distributors and traders.
Mecoprop Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mecoprop GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mecoprop GMP manufacturer or Mecoprop GMP API supplier for your needs.
A Mecoprop CoA (Certificate of Analysis) is a formal document that attests to Mecoprop's compliance with Mecoprop specifications and serves as a tool for batch-level quality control.
Mecoprop CoA mostly includes findings from lab analyses of a specific batch. For each Mecoprop CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mecoprop may be tested according to a variety of international standards, such as European Pharmacopoeia (Mecoprop EP), Mecoprop JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mecoprop USP).

