



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
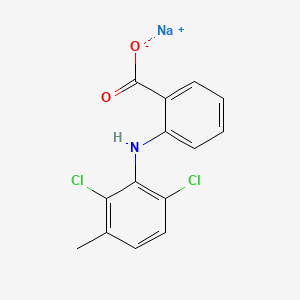

1. Benzoic Acid, 2-((2,6-dichloro-3-methylphenyl)amino)-, Monosodium Salt, Monohydrate
2. Meclofenamate
3. Meclofenamate Sodium Anhydrous
4. Meclofenamate Sodium Monohydrate
5. Meclofenamate, Sodium
6. Meclofenamic Acid
7. Meclomen
8. Sodium Meclofenamate
1. 6385-02-0
2. Meclomen
3. Meclofenamic Acid Sodium Salt
4. Meclonax
5. Movens
6. Sodium Meclofenamate
7. Meclofenamic Acid Sodium
8. Meclofenamic Acid (sodium)
9. Meclodium
10. Sodium 2-((2,6-dichloro-3-methylphenyl)amino)benzoate
11. Meclofenamate (sodium)
12. 9mmq0yer4e
13. 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic Acid Sodium Salt
14. Sodium;2-(2,6-dichloro-3-methylanilino)benzoate
15. Mls000069578
16. Chebi:6711
17. Benzoic Acid, 2-((2,6-dichloro-3-methylphenyl)amino)-, Monosodium Salt
18. Smr000058785
19. Meclomen (tn)
20. Sodium Meclophenamate
21. Chembl876
22. Cl-583.na Salt
23. Meclofenamate Sodium Anhydrous
24. Nsc-757088
25. Benzoic Acid, 2-((2,6-dichloro-3-methylphenyl)amino)-, Monosodium Salt, Monohydrate
26. Einecs 228-983-3
27. Unii-9mmq0yer4e
28. Ci 583
29. Mfcd00077376
30. Meclofenamate Sodium [usan:usp]
31. Monosodium N-(2,6-dichloro-m-tolyl)anthranilate
32. N-(2,6-dichloro-m-tolyl)anthranilic Acid Sodium Salt
33. Benzoic Acid, 2-((2,6-dichloro-3-methylphenyl)amino)-, Sodium Salt (1:1)
34. Opera_id_596
35. Anthranilic Acid, N-(2,6-dichloro-m-tolyl)-, Monosodium Salt
36. Lopac-m-4531
37. Mls001056523
38. Mls001077271
39. Schembl1649454
40. Dtxsid8045567
41. Sodium Meclofenamate (anhydrous)
42. Hms501j06
43. Hms2091b08
44. Hms2231h18
45. Hms3259f13
46. Hms3262a16
47. Hms3372d22
48. Hms3649o09
49. Hms3652l10
50. Hms3885h19
51. Hy-b1320
52. Tox21_500727
53. Ccg-40117
54. S4295
55. Meclofenamate Sodium [who-dd]
56. Akos009157878
57. Ccg-267676
58. Lp00727
59. Nc00649
60. Nsc 757088
61. Ncgc00016169-01
62. Ncgc00016169-02
63. Ncgc00016169-03
64. Ncgc00094073-01
65. Ncgc00094073-02
66. Ncgc00261412-01
67. Db-054565
68. 2-(2,6-dichloro-3-methyl-anilino)benzoate
69. Cs-0013081
70. Eu-0100727
71. Ft-0603690
72. M1269
73. Sw219723-1
74. En300-51912
75. A12520
76. C02996
77. D08162
78. D81964
79. M 4531
80. Q27107307
81. Sodium 2-(2,6-dichloro-3-methylphenylamino)benzoate
82. Sodium2-((2,6-dichloro-3-methylphenyl)amino)benzoate
83. 2-([2,6-dichloro-3-methylphenyl]amino)benzoic Acid Sodium
| Molecular Weight | 318.1 g/mol |
|---|---|
| Molecular Formula | C14H10Cl2NNaO2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 316.9986282 g/mol |
| Monoisotopic Mass | 316.9986282 g/mol |
| Topological Polar Surface Area | 52.2 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 332 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Meclofenamate sodium |
| PubMed Health | Meclofenamate Sodium (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Meclofenamate sodium, USP is N-(2,6-dichloro-m-tolyl) anthranilic acid, sodium salt, monohydrate. It is an anti-inflammatory drug for oral administration. Meclofenamate sodium capsules, USP contain 50 mg or 100 mg meclofenamic acid as the sodium salt... |
| Active Ingredient | Meclofenamate sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base |
| Market Status | Prescription |
| Company | Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Meclofenamate sodium |
| PubMed Health | Meclofenamate Sodium (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Meclofenamate sodium, USP is N-(2,6-dichloro-m-tolyl) anthranilic acid, sodium salt, monohydrate. It is an anti-inflammatory drug for oral administration. Meclofenamate sodium capsules, USP contain 50 mg or 100 mg meclofenamic acid as the sodium salt... |
| Active Ingredient | Meclofenamate sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 50mg base |
| Market Status | Prescription |
| Company | Mylan |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)






