


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
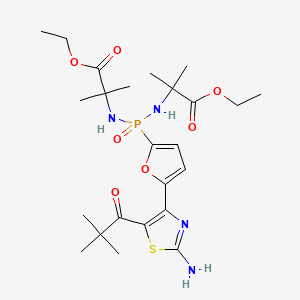

1. 882757-24-6
2. Mb-07803
3. Mb07803
4. Mb 07803
5. Gg81xf45c2
6. Ethyl 2-[[[5-[2-amino-5-(2,2-dimethylpropanoyl)-1,3-thiazol-4-yl]furan-2-yl]-[(1-ethoxy-2-methyl-1-oxopropan-2-yl)amino]phosphoryl]amino]-2-methylpropanoate
7. Unii-gg81xf45c2
8. Chembl4297400
9. Schembl14255484
10. Dtxsid90236932
11. Act04837
12. Db05053
13. Sb17400
14. Vk-0612
15. Hy-16309
16. Cs-0006389
17. Q27279094
18. Alanine, N,n'-((5-(2-amino-5-(2,2-dimethyl-1-oxopropyl)-4-thiazolyl)-2-furanyl)phosphinylidene)bis(2-methyl-, 1,1''-diethyl Ester
19. N,n'-((5-(2-amino-5-(2,2-dimethyl-1-oxopropyl)-4-thiazolyl)-2-furanyl)phosphinylidene)bis(2-methylalanine) Diethyl Ester
| Molecular Weight | 556.6 g/mol |
|---|---|
| Molecular Formula | C24H37N4O7PS |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 14 |
| Exact Mass | 556.21205770 g/mol |
| Monoisotopic Mass | 556.21205770 g/mol |
| Topological Polar Surface Area | 191 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 872 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in diabetes mellitus type 2.
MB07803 is a selective inhibitor of fructose-1, 6-bisphosphatase (FBPase), a regulatory enzyme in the pathway responsible for the production of glucose in the liver, known as the gluconeogenesis pathway. By specifically inhibiting this pathway, liver glucose production should be reduced and blood sugar levels decreased in patients with diabetes, independent of insulin levels and body weight.


