



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
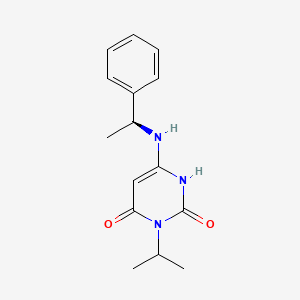

1. Myk-461
1. Camzyos
2. 1642288-47-8
3. Mavacamten [inn]
4. Mavacamten [usan]
5. Mavacamten [who-dd]
6. Myk-461
7. 6-[[(1s)-1-phenylethyl]amino]-3-propan-2-yl-1h-pyrimidine-2,4-dione
8. Qx45b99r3j
9. Sar-439152
10. (s)-3-isopropyl-6-((1-phenylethyl)amino)pyrimidine-2,4(1h,3h)-dione
11. Sar439152
12. 2,4(1h,3h)-pyrimidinedione, 3-(1-methylethyl)-6-(((1s)-1-phenylethyl)amino)-
13. 2,4(1h,3h)-pyrimidinedione, 3-(1-methylethyl)-6-[[(1s)-1-phenylethyl]amino]-
14. Mavacamten (myk-461)
15. Unii-qx45b99r3j
16. Myk461
17. Chembl4297517
18. Schembl16320785
19. Gtpl11265
20. Ex-a2072
21. Bdbm50575174
22. S8861
23. Who 10492
24. Db14921
25. Myk-461; Mavacamten; Sar439152
26. Sar-439152; Myk-461
27. Hy-109037
28. Cs-0031211
29. E83742
30. (s)-3-isopropyl-6-((1-phenylethyl)amino) Pyrimidine-2,4(1h,3h)-dione
31. (s)-3-isopropyl-6-((1-phenylethyl)amino)pyrimidine-2, 4(1h,3h)-dione
32. (s)-3-isopropyl-6-(1-phenylethylamino)pyrimidine-2,4(1h,3h)-dione
33. 3-(1-methylethyl)-6-[[(1s)-1-phenylethyl]amino]-2,4(1h,3h)-pyrimidinedione
34. 6-(((1s)-1-phenylethyl)amino)-3-(propan-2-yl)-1,2,3,4 Tetrahydropyrimidine-2,4-dione
35. 6-{[(1s)-1-phenylethyl]amino}-3-(propan-2-yl)pyrimidine-2,4(1h,3h)-dione
| Molecular Weight | 273.33 g/mol |
|---|---|
| Molecular Formula | C15H19N3O2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 273.147726857 g/mol |
| Monoisotopic Mass | 273.147726857 g/mol |
| Topological Polar Surface Area | 61.4 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of hypertrophic cardiomyopathy


