



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
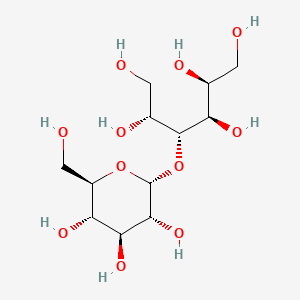

1. 585-88-6
2. D-maltitol
3. Maltisorb
4. Amalty Mr 100
5. 4-o-alpha-d-glucopyranosyl-d-glucitol
6. Malbit
7. Maltit
8. Amalti Syrup
9. Malti Mr
10. D-glucitol, 4-o-a-d-glucopyranosyl-
11. Lycasin Hbc
12. Maltitol Solution
13. Hydrogenated Maltose
14. Maltisweet 3145
15. Dried Maltitol Syrup
16. Sweetpearl P 200
17. Maltitol Syrup Powder
18. Cerestar 16303
19. Malbit Ch 16385
20. Maltitol (d-maltitol)
21. Maltidex Ch 16385
22. D-4-o-alpha-d-glucopyranosylglucitol
23. Amalty
24. Mls000069503
25. D65dg142wk
26. (2s,3r,4r,5r)-4-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,5,6-pentol
27. Ins No.965(i)
28. Chebi:68428
29. Ins-965(i)
30. E-965(i)
31. Smr000058608
32. Alpha-d-glucopyranosyl-(1->4)-d-glucitol
33. (2s,3r,4r,5r)-4-(((2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexane-1,2,3,5,6-pentaol
34. Maltitol [ban:nf]
35. Mfcd00006600
36. Unii-d65dg142wk
37. Delta-maltitol
38. Hsdb 7971
39. Maltitol (nf)
40. Einecs 209-567-0
41. Brn 0089983
42. Maltitol, >=98%
43. Maltitol [inci]
44. Opera_id_636
45. Maltitol [fcc]
46. Maltitol [ii]
47. Maltitol [mart.]
48. Maltitol [usp-rs]
49. D-glucopyranosyl-d-glucitol
50. Schembl15108
51. 4-o-alpha-d-glucopyranosyl-
52. Manganese(iv)oxide,activated
53. 5-17-07-00145 (beilstein Handbook Reference)
54. Mls001148576
55. Chembl63558
56. Maltitol Solution [nf]
57. Maltitol [ep Monograph]
58. Dtxsid0044444
59. 4-o-a-glucopyranosyl-d-sorbitol
60. Hms2234b21
61. Alpha-d-glc-(1->4)-d-glc-ol
62. Hy-b2122
63. Zinc4262249
64. Alpha-d-glcp-(1->4)-d-glc-ol
65. S3950
66. Alpha-d-glucosyl-(1->4)-d-glucitol
67. Ccg-267963
68. Smp1_000186
69. Cs-15369
70. D-glucitol, 4-o-alpha-d-glucopyranosyl-
71. E965
72. Cs-0020280
73. D-glucitol, 4--o-.alpha.-d-glucopyranosyl
74. Delta-4-o-alpha-delta-glucopyranosylglucitol
75. Glucitol, 4-o-alpha-d-glucopyranosyl-, D-
76. M0601
77. M0797
78. 4-o-alpha-delta-glucopyranosyl-delta-glucitol
79. D04845
80. Q423882
81. Sr-01000759231
82. Q-101038
83. Sr-01000759231-4
84. Hydrogenated High Maltose-content Glucose Syrup
85. Maltitol, European Pharmacopoeia (ep) Reference Standard
86. Maltitol, United States Pharmacopeia (usp) Reference Standard
87. Maltitol, Pharmaceutical Secondary Standard; Certified Reference Material
88. Wurcs=2.0/2,2,1/[h2122h][a2122h-1a_1-5]/1-2/a4-b1
89. (2s,3r,4r,5r)-4-((2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yloxy)hexane-1,2,3,5,6-pentaol
90. (2s,3r,4r,5r)-4-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxyhexane-1,2,3,5,6-pentol
91. (2s,3r,4r,5r)-4-{[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexane-1,2,3,5,6-pentol
92. (2s,3r,4r,5r)-4-{[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl]oxy}hexane-1,2,3,5,6-pentol
| Molecular Weight | 344.31 g/mol |
|---|---|
| Molecular Formula | C12H24O11 |
| XLogP3 | -5.2 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 344.13186158 g/mol |
| Monoisotopic Mass | 344.13186158 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sugar Alcohols; Maltose/analogs & derivatives; Sweetening Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Chemical changes in the blood induced by maltitol were compared with those induced by glucose in both healthy people and patients with several disorders, including diabetes mellitus. Blood glucose levels of healthy subjects were determined after the administration of glucose (12.5, 25, or 50 g) or maltitol (50 g). Based on the glucose absorption curve, 38% of the maltitol that was orally administered was absorbed through the intestinal tract, but the absorption of maltitol was more delayed than that of glucose.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: 603. Hydrogenated Glucose Syrups (1985). Available from, as of July 20, 2011: https://www.inchem.org/pages/jecfa.html
Pieces of New Zealand rabbit small intestine were everted and incubated with 100 mM substrate (maltitol, sucrose, or glucose). After removal at different times (20, 40, or 60 min.) of incubation, the volume of serosal fluid and the dry mass of the gut pieces were determined. Maltitol was hydrolyzed, and the hydrolysis products were absorbed by the everted sacs. No maltitol was detected in the serosal fluid. The serosal glucose concentration increased at a slower rate after incubation with maltitol than after incubation with glucose or sucrose. The rate of hydrolysis and absorption decreased with the longer time of incubation.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: 603. Hydrogenated Glucose Syrups (1985). Available from, as of July 20, 2011: https://www.inchem.org/pages/jecfa.html
In an in vitro study of (14)C-U-maltitol in everted intestinal sacs, the highest transport of (14)C-maltitol was displayed in the jejunum, followed by the ileum and duodenum. Twenty-four hr after oral administration of (14)C-U-maltitol, 60% of the radioactivity was detected in the cecum, large intestine, and feces. Five percent was excreted in the urine and 1.2% was expired as CO2 within 24 hr. When (14)C-U-maltitol was injected i.v., over 35, 60, and 85% of the administered dose was excreted in the urine within 1, 3, and 24 hr, respectively.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: 603. Hydrogenated Glucose Syrups (1985). Available from, as of July 20, 2011: https://www.inchem.org/pages/jecfa.html
Two male beagle dogs were given maltitol-U-(14)C (51.2 uCi) by stomach tube. Blood samples were collected until 32 hr after dosing. The peak radiolabel concentration in plasma was 2 hr after maltitol administration (304 and 263 ug/mL, expressed as maltitol equivalent in the 2 dogs). The radioactivity present in the urine after 48 hr was 7.8 and 3.8% of the administered dose in the 2 animals.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: 603. Hydrogenated Glucose Syrups (1985). Available from, as of July 20, 2011: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for Maltitol (11 total), please visit the HSDB record page.
The metabolism of maltitol (4-alpha-D-glucosylsorbitol) was assessed in fasting conventional (C) rats, C mice and germ-free (GF) mice, using [U-14C]maltitol. The radiorespirometric patterns of (14)CO2 collected for 48 hr after the administration of labelled maltitol were characterized by a constant rate of (14)CO2 production lasting 4 hr for both C rats and mice. The pattern for the GF mice showed a peak at the second hour followed immediately by a slow decrease. The percentage recovery of (14)CO2 was significantly lower for the GF mice (59%) compared with C animals (72-74%). Urine, feces and intestinal contents after 48 hr totalled 19% of the administered radioactivity in the C rats and mice and 39% in the GF mice. The digestibility of maltitol and the absorption of sorbitol in GF mice was also assessed. The cecum and small intestine of GF mice, 3 hr after administration of equimolar quantities of maltitol (140 mg/kg body-weight) or sorbitol (70 mg/kg body-weight), contained 39 and 51% of the ingested dose respectively, present mostly in the cecum as sorbitol. The alpha-glucosidase (maltase) activity of the small intestine was appreciably higher (1.5-1.7 times) in the GF mice than in the C mice. These results suggest that the enzymic activities in the small intestine of mice and rats are sufficient to hydrolyse maltitol extensively. Consequently, the slow absorption of sorbitol seems to be an important factor limiting the overall assimilation of maltitol in the small intestine.
PMID:2107869 Wursch P et al; Br J Nutr 63 (1): 7-15 (1990)
Conventional (CV) rats were given a single oral dose of 1 or 2 g maltitol. Urine and faeces were collected during the following 24 hr and their contents of maltitol and sorbitol were measured. Very little of either substance appeared in the faeces but appreciable amounts of sorbitol found in the urine indicated that the maltitol had been hydrolysed. Excretion of maltitol and sorbitol was compared in germ-free and CV rats given an oral dose of 2 g maltitol. Significantly less of both substances was recovered in the faeces of CV rats, but urinary excretion was similar in both environments. Maltitol injected intravenously gave rise to only traces of sorbitol in the excreta. A dose of 250 mg was cleared almost completely from the circulation within 1 hr. It is concluded that maltitol is hydrolysed by animal tissues, either in the gut lumen before absorption or in the gut wall during absorption. Maltitol and sorbitol are also degraded by gut bacteria, mostly in sites distal to the main absorptive area. The contribution to the host's nutrition would depend on the extent to which the end-products of fermentation are absorbed from the colon.
PMID:7171535 Lian-Loh R et al; Br J Nutr 48 (3): 477-81 (1982)
Weanling Wistar rats placed on diets containing 13 or 26% maltitol for 9 weeks had reduced body-weight gains and increased intestinal weights as compared with controls. Enzymatic tests in dosed rats indicated that the alpha-glycosidic linkage of maltitol was not hydrolysed with pancreatic enzymes or enzymes of the intestinal mucosa. Maltitol dehydrogenase was not observed in liver-cell cytoplasm and prolonged maltitol administration did not induce hepatic sorbitol dehydrogenase.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: 603. Hydrogenated Glucose Syrups (1985). Available from, as of July 20, 2011: https://www.inchem.org/pages/jecfa.html
... Reports from authoritative bodies and reviews indicates that the decrease in pH in plaque as a consequence of metabolic acid production by saccharolytic bacteria when exposed to fermentable carbohydrates (i.e. sugars and starches) may promote demineralization and prevent remineralization of the hydroxyapatite crystals. Tooth hydroxyapatite crystals are very resistant to dissolution at neutral pH, but their solubility drastically increases as pH drops. Typically, the critical pH for dental enamel is around 5.5. ... Demineralization of tooth tissues can also occur as a result of consumption of dietary acids in foods or beverages, and that frequent consumption can lead to dental erosion. Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose are slowly metabolized by bacteria in the mouth. The rate and amount of acid production from these food constituents is significantly less than that from sucrose. ... Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose do not promote dental caries because they do not lower plaque pH to the level associated with enamel demineralization. ... A cause and effect relationship has been established between the consumption of sugar-containing foods/drinks at an exposure frequency of four times daily or more and an increased tooth demineralization, and that the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose, instead of sugar in sugar-containing foods/drinks, may maintain tooth mineralization by decreasing tooth demineralization compared with sugar-containing foods, provided that such foods/drinks do not lead to dental erosion.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm
The food constituents xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose resulted in reduced post-prandial blood glucose (or insulinemic) responses compared with sugars on a weight by weight basis owing to their reduced/delayed digestion/absorption and/or to a decrease in the amount of available carbohydrates, and that the consumption of foods/drinks in which xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose replaced sugars induced lower post-prandial glycemic and insulinemic responses than sugar-containing foods/drinks. ... A cause and effect relationship has been established between the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose instead of sugar and reduction in post-prandial blood glucose responses (without disproportionally increasing post-prandial insulinemic responses) as compared to sugar-containing foods/drinks.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm


