



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
0

1. Pm 01183
2. Pm-01183
3. Pm01183
1. 497871-47-3
2. Pm01183
3. Tryptamicidin
4. Zepzelca
5. Pm-01183
6. Pm-1183
7. 2cn60tn6zs
8. Pm 01183
9. Pm1183
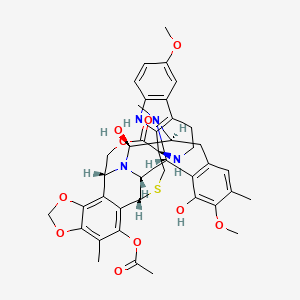
10. [(1r,2r,3r,11s,12s,14r,26r)-5,12-dihydroxy-6,6'-dimethoxy-7,21,30-trimethyl-27-oxospiro[17,19,28-trioxa-24-thia-13,30-diazaheptacyclo[12.9.6.13,11.02,13.04,9.015,23.016,20]triaconta-4(9),5,7,15,20,22-hexaene-26,1'-2,3,4,9-tetrahydropyrido[3,4-b]indole]-22-yl] Acetate
11. Zepsyre
12. Lurbinectedin [inn]
13. Unii-2cn60tn6zs
14. Lurbinectedin [mi]
15. Lurbinectedin [usan:inn]
16. Lurbinectedin [usan]
17. Lurbinectedin [who-dd]
18. Chembl4297516
19. Schembl16152477
20. Gtpl10681
21. Dtxsid30198065
22. Lurbinectedin [orange Book]
23. Ex-a4316
24. Who 9397
25. Nsc826275
26. S9603
27. At22223
28. Cs-6323
29. Db12674
30. Nsc-826275
31. Hy-16293
32. J3.531.659k
33. J3.652.626b
34. Pm01183;pm-1183;ly-01017;ryptamicidin
35. Q27254568
36. (1'r,6r,6ar,7r,13s,14s,16r)-8,14-dihydroxy-6',9-dimethoxy-4,10,23-trimethyl-19-oxo-2',3',4',6,7,9',12,13,14,16-decahydro-6ahspiro(7,13-azano-6,16-(epithiopropanooxymethano)(1,3)dioxolo(7,8)isoquinolino(3,2-b)(3)benzazocine-20,1'-pyrido(3,4-b)indol)-5-yl Acetate
37. [(1r,2r,3r,11s,12s,14r,26r)-5,12-dihydroxy-6,6'-dimethoxy-7,21,30-trimethyl-27-oxospiro[17,19,28-trioxa-24-thia-13,30-diazaheptacyclo[12.9.6.13,11.02,13.04,9.015,23.016,20]triaconta-4(9),5,7,15,20,22-hexaene-26,1'-2,3,4,9-tetrahydropyrido[3,4-b]indole]-22
38. Spiro(6,16-(epithiopropanoxymethano)-7,13-imino-12h-1,3-dioxolo(7,8)isoquino(3,2-b)(3)benzazocine-20,1'-(1h)pyrido(3,4-b)indol)-19-one, 5-(acetyloxy)-2',3',4',6,6a,7,9',13,14,16-decahydro-8,14-dihydroxy-6',9-dimethoxy-4,10,23-trimethyl-, (1'r,6r,6ar,7r,13s,14s,16r)-
| Molecular Weight | 784.9 g/mol |
|---|---|
| Molecular Formula | C41H44N4O10S |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 4 |
| Exact Mass | 784.27781479 g/mol |
| Monoisotopic Mass | 784.27781479 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 56 |
| Formal Charge | 0 |
| Complexity | 1530 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lurbinectedin is indicated for the treatment of adult patients with metastatic small-cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
Treatment of small cell lung cancer
Lurbinectedin exerts its chemotherapeutic activity by covalently binding to DNA, resulting in double-strand DNA breaks and subsequent cell death. Lurbinectedin has been associated with myelosuppression, and patients receiving therapy with this agent should be closely monitored for evidence of cytopenias. Prior to beginning therapy, ensure baseline neutrophil counts are >1,500 cells/mm3 and platelet counts are >100,000/mm3. The supplementary use of granulocyte colony-stimulating factor (G-CSF) should be considered if the neutrophil count falls below 500 cells/mm3. Lurbinectedin has also been associated with hepatotoxicity. Monitor liver function tests at baseline and regular intervals throughout therapy, and consider holding, reducing, or permanently discontinuing therapy based on the severity of observed hepatotoxicity.
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX69 - Lurbinectedin
Absorption
Following intravenous administration, the Cmax and AUC0-inf were 107 g/L and 551 g*h/L, respectively. No accumulation between dosing intervals (every 3 weeks) has been observed. No significant differences in absorption were found between special populations (e.g. based on age, sex, ethnicity, etc.), but lurbinectedin has not been studied in the setting of severe renal impairment or moderate/severe hepatic impairment.
Route of Elimination
Approximately 89% of a given dose is recovered in the feces (<0.2% unchanged) and 6% in the urine (1% unchanged).
Volume of Distribution
The steady-state volume of distribution of lurbinectedin is 504 L.
Clearance
The total plasma clearance of lurbinectedin is approximately 11 L/h.
Lurbinectedin is metabolized primarily by CYP3A4 _in vitro_, though specific data regarding its biotransformation are lacking. An N-desmethylated metabolite has been identified in canine subjects.
The terminal half-life of lurbinectedin is 51 hours.
Lurbinectedin is a DNA alkylating agent. It covalently binds to guanine residues in the DNA minor groove, forming adducts that bend the DNA helix towards the major groove. This process triggers a cascade of events that affect the activity of transcription factors and impairs DNA repair pathways, ultimately leading to double-strand DNA breaks and eventual cell death. Additional mechanism(s) of action include inhibition of RNA-polymerase-II activity, inactivation of Ewing Sarcoma Oncoprotein (EWS-FL11) via nuclear redistribution, and the inhibition of human monocyte activity and macrophage infiltration into tumor tissue.


