


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
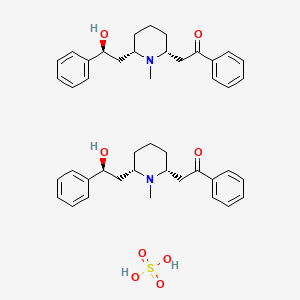

1. Lobeline
2. Smokeless
3. Sulfate, Lobeline
1. 134-64-5
2. Lobeline Sulphate
3. Lobelin Sulphate
4. 4cj480v2hp
5. Bantron
6. Lobeline (sulfate)
7. Lobelin Sulfate
8. 2-[(2r,6s)-6-[(2s)-2-hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl]-1-phenylethanone;sulfuric Acid
9. 2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2-yl)-1-phenylethanone Sulfate
10. Einecs 205-151-8
11. Unii-4cj480v2hp
12. Smokeless (tn)
13. Lobeline, Sulfate (2:1) (salt)
14. Inflatine Sulfate
15. Inflatine Sulphate
16. Lobeline Sulfate, Aldrichcpr
17. Lobeline Sulfate [mi]
18. Schembl3686934
19. .alpha.-lobeline Sulfate
20. .alpha.-lobeline Sulphate
21. Dtxsid80158452
22. Lobeline Sulfate [mart.]
23. Lobeline Sulfate [who-dd]
24. Hms3886l05
25. Mfcd00135592
26. S5844
27. Akos032428277
28. Ethanone, 2-(6-(2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenyl-, (2theta-(2alpha,6alpha(s)))-, Sulfate (2:1) (salt)
29. Hy-128420
30. Cs-0099255
31. D08138
32. J-006568
33. Q27259407
34. 2-(6-(.beta.-hydroxyphenethyl)-1-methyl-2-piperidyl)acetophenone Sulfate
35. 2-(6-(.beta.-hydroxyphenethyl)-1-methyl-2-piperidyl)acetophenone Sulphate
36. 2-((2r,6s)-6-((2s)-2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenylethanone Sulfate
37. 2-((2r,6s)-6-((2s)-2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenylethanone Sulphate
38. 2-((2r,6s)-6-((s)-2-hydroxy-2-phenylethyl)-1-methylpiperidin-2-yl)-1-phenylethanone Hemisulfate
| Molecular Weight | 773.0 g/mol |
|---|---|
| Molecular Formula | C44H56N2O8S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 772.37573792 g/mol |
| Monoisotopic Mass | 772.37573792 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 55 |
| Formal Charge | 0 |
| Complexity | 494 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Respiratory System Agents
Drugs used for their effects on the respiratory system. (See all compounds classified as Respiratory System Agents.)
Nicotinic Agonists
Drugs that bind to and activate nicotinic cholinergic receptors (RECEPTORS, NICOTINIC). Nicotinic agonists act at postganglionic nicotinic receptors, at neuroeffector junctions in the peripheral nervous system, and at nicotinic receptors in the central nervous system. Agents that function as neuromuscular depolarizing blocking agents are included here because they activate nicotinic receptors, although they are used clinically to block nicotinic transmission. (See all compounds classified as Nicotinic Agonists.)
Ganglionic Stimulants
Agents that mimic neural transmission by stimulation of the nicotinic receptors on postganglionic autonomic neurons. Drugs that indirectly augment ganglionic transmission by increasing the release or slowing the breakdown of acetylcholine or by non-nicotinic effects on postganglionic neurons are not included here nor are the nonspecific cholinergic agonists. (See all compounds classified as Ganglionic Stimulants.)


