



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
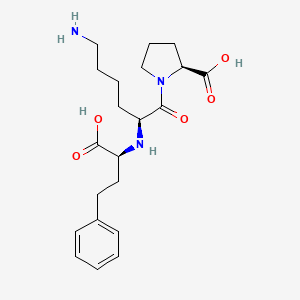

1. Lisinopril Maleate (1:1)
2. Lisinopril Sulfate (1:2)
3. Lysinopril
4. Mk-521
5. Prinivil
6. Zestril
1. Prinivil
2. Zestril
3. 76547-98-3
4. Linopril
5. Lisipril
6. Lysinopril
7. Tensopril
8. 83915-83-7
9. Lisinopril Anhydrous
10. Acercomp
11. Inhibril
12. Noperten
13. Presiten
14. Sinopril
15. Acerbon
16. Carace
17. Vivatec
18. Coric
19. Alapril
20. Inopril
21. Lisinal
22. Lisoril
23. Lispril
24. Novatec
25. Sinopryl
26. Cipral
27. Cipril
28. Linvas
29. Lipril
30. Loril
31. Prinil
32. Tensyn
33. Tersif
34. Lisinoprilum
35. Longes
36. Hipril
37. Mk-521
38. Lisinopril (inn)
39. Lisinopril [inn]
40. [n2-[(s)-1-carboxy-3-phenylpropyl]-l-lysyl-l-proline
41. Lisinopril (zestril)
42. N2-[(1s)-1-carboxy-3-phenylpropyl]-l-lysyl-l-proline
43. Zestril (tn)
44. 7q3p4bs2fd
45. Chebi:43755
46. Qbrelis
47. Nsc-751176
48. Nsc-758151
49. (s)-1-((s)-6-amino-2-(((s)-1-carboxy-3-phenylpropyl)amino)hexanoyl)pyrrolidine-2-carboxylic Acid
50. Lpr
51. L-proline, N2-((1s)-1-carboxy-3-phenylpropyl)-l-lysyl-
52. (s)-1-(n2-(1-carboxy-3-phenylpropyl)-l-lysyl)-l-proline
53. Nanopril
54. Lisinoprilum [latin]
55. (s)-1-(n(2)-(1-carboxy-3-phenylpropyl)-l-lysyl)-l-proline
56. Chembl1237
57. (2s)-1-[(2s)-6-amino-2-[[(1s)-1-carboxy-3-phenylpropyl]amino]hexanoyl]pyrrolidine-2-carboxylic Acid
58. Lizinopril
59. Diroton
60. Lisitec
61. Lisopril
62. Listril
63. Lizonoton
64. Optimon
65. Prinvil
66. Skopryl
67. Vitopril
68. Amicor
69. Doneka
70. Irumed
71. Laaven
72. Mls002154258
73. Mk 521
74. Mk 522
75. (2s)-1-[(2s)-6-amino-2-{[(1s)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic Acid
76. Smr000544473
77. Ccris 3568
78. Smr001233519
79. Einecs 278-488-1
80. Unii-7q3p4bs2fd
81. Brn 4276619
82. Lysinopryl
83. (s)-1-[n2-(1-carboxy-3-phenylpropyl)-l-lysyl]-l-proline
84. L-proline, N2-[(1s)-1-carboxy-3-phenylpropyl]-l-lysyl-
85. N-(1(s)-carboxy-3-phenylpropyl)-l-lysyl-l-proline
86. N2-((s)-1-carboxy-3-phenylpropyl)-l-lysyl-l-proline
87. Lisiprilprinivil
88. Hsdb 6852
89. (s)-1-(n(sup 2)-(1-carboxy-3-phenylpropyl)-l-lysyl)-l-proline
90. 77726-95-5
91. (s)-1-[n2-(1-carboxy-3-phenylpropyl)-l-lysyl]-l-proline Dihydrate
92. 1-(n2-(1-carboxy-3-phenylpropyl)-l-lysyl)-l-proline
93. Spectrum_000497
94. Lisinopril [mi]
95. Prestwick0_000301
96. Prestwick1_000301
97. Prestwick2_000301
98. Prestwick3_000301
99. Spectrum2_001456
100. Spectrum3_000941
101. Spectrum4_001040
102. Spectrum5_000995
103. (s)-lisinopril Dihydrate
104. Lisinopril [who-dd]
105. Schembl15680
106. Bspbio_000262
107. Kbiogr_001599
108. Kbioss_000977
109. (2s)-1-[(2s)-6-amino-2-[[(2s)-1-hydroxy-1-oxo-4-phenylbutan-2-yl]amino]hexanoyl]pyrrolidine-2-carboxylic Acid
110. Mls001306436
111. Mls001306481
112. Bidd:gt0755
113. Divk1c_001037
114. Spectrum1501217
115. Spbio_001351
116. Spbio_002481
117. Bpbio1_000290
118. Gtpl6360
119. Ici-209k
120. Dtxsid6040537
121. Bdbm66979
122. Hms503o15
123. Kbio1_001037
124. Kbio2_000977
125. Kbio2_003545
126. Kbio2_006113
127. Kbio3_002002
128. L-proline, 1-(n2-(1-carboxy-3-phenylpropyl)-l-lysyl)-
129. N(2)-[(1s)-1-carboxy-3-phenylpropyl]-l-lysyl-l-proline
130. N~2~-[(1s)-1-carboxy-3-phenylpropyl]-l-lysyl-l-proline
131. Cid_22887897
132. Ninds_001037
133. Hms1921b14
134. Hms2090o14
135. Hms2092l21
136. Hms2850n20
137. Pharmakon1600-01501217
138. 1-[nalpha-[(s)-1-carboxy-3-phenylpropyl]-l-lysyl]-l-proline
139. L-proline, 1-(n2-(1-carboxy-3-phenylpropyl)-l-lysyl)-, (s)-
140. Zinc3812863
141. Bdbm50367879
142. Ccg-39190
143. Dl-434
144. Nsc751176
145. Nsc758151
146. S2076
147. Akos015836369
148. Akos015894970
149. L-proline, 1-(n(sup 2)-(1-carboxy-3-phenylpropyl)-l-lysyl)-, (s)-
150. Bcp9000856
151. Db00722
152. Idi1_001037
153. Ncgc00179623-01
154. Ncgc00179623-07
155. Hy-18206
156. Sbi-0051692.p002
157. L0220
158. C76449
159. D08131
160. Ab00052250-13
161. Ab00052250_14
162. Ab00052250_15
163. 547l983
164. A838743
165. Q412208
166. Sr-05000001786
167. Sr-05000001786-1
168. Brd-k67966701-335-03-5
169. Z2786051707
170. N-[n2 (1(s)-carboxy-3-phenylpropyl)-l-lysyl]-l-proline
171. N-[n2 -(1(s)-carboxy-3-phenylpropyl)-l-lysyl]-l-proline
172. 1-(n(sup 2)-((s)-1-carboxy-3-phenylpropyl)-l-lysyl)-l-proline
173. L-proline, 1-(n(sup 2)-(1-carboxy-3-phenylpropyl)-l-lysyl)-
174. (2s)-1-[(2s)-6-amino-2-[[(1s)-1-carboxy-3-phenyl-propyl]amino]hexanoyl]proline;hydrate
175. (2s)-1-[(2s)-6-amino-2-[[(1s)-1-carboxy-3-phenyl-propyl]amino]hexanoyl]pyrrolidine-2-carboxylic Acid;lisinopril
176. (2s)-1-[(2s)-6-amino-2-[[(1s)-1-carboxy-3-phenylpropyl]amino]-1-oxohexyl]-2-pyrrolidinecarboxylic Acid;hydrate
177. (2s)-1-[(2s)-6-amino-2-[[(1s)-1-carboxy-3-phenylpropyl]amino]hexanoyl]pyrrolidine-2-carboxylic Acid;hydrate
178. (2s)-1-[(2s)-6-azanyl-2-[[(2s)-1-oxidanyl-1-oxidanylidene-4-phenyl-butan-2-yl]amino]hexanoyl]pyrrolidine-2-carboxylic Acid;hydrate
179. (s)-1-((s)-6-amino-2-((s)-1-carboxy-3-phenylpropylamino)hexanoyl)pyrrolidine-2-carboxylic Acid
| Molecular Weight | 405.5 g/mol |
|---|---|
| Molecular Formula | C21H31N3O5 |
| XLogP3 | -2.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 12 |
| Exact Mass | 405.22637110 g/mol |
| Monoisotopic Mass | 405.22637110 g/mol |
| Topological Polar Surface Area | 133 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Lisinopril |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-Carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O52H... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Hikma Intl Pharms; Wockhardt; Ranbaxy; Apotex; Accord Hlthcare; Aurobindo; Lupin; Sandoz; Prinston; Invagen Pharms; Watson Labs; Ivax Sub Teva Pharms; Vintage; Mylan |
| 2 of 6 | |
|---|---|
| Drug Name | Prinivil |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | PRINIVIL (Lisinopril), a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
| 3 of 6 | |
|---|---|
| Drug Name | Zestril |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5.2... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 4 of 6 | |
|---|---|
| Drug Name | Lisinopril |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-Carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O52H... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Hikma Intl Pharms; Wockhardt; Ranbaxy; Apotex; Accord Hlthcare; Aurobindo; Lupin; Sandoz; Prinston; Invagen Pharms; Watson Labs; Ivax Sub Teva Pharms; Vintage; Mylan |
| 5 of 6 | |
|---|---|
| Drug Name | Prinivil |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | PRINIVIL (Lisinopril), a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
| 6 of 6 | |
|---|---|
| Drug Name | Zestril |
| PubMed Health | Lisinopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5.2... |
| Active Ingredient | Lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 30mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Astrazeneca |
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents; Cardiotonic Agents
National Library of Medicine's Medical Subject Headings. Lisinopril. Online file (MeSH, 2016). Available from, as of October 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
Prinivil is indicated for the treatment of hypertension in adult patients and pediatric patients 6 years of age and older to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. ... Prinivil may be administered alone or with other antihypertensive agents /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Prinivil is indicated to reduce signs and symptoms of heart failure in patients who are not responding adequately to diuretics and digitalis /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Prinivil is indicated for the reduction of mortality in treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers. /Included in US product label/
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
For more Therapeutic Uses (Complete) data for LISINOPRIL (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Prinivil as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Milk of lactating rats contains radioactivity following administration of (14)C lisinopril. It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ACE inhibitors, discontinue nursing or discontinue Prinivil.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Antihypertensive effects and safety of Prinivil have been established in pediatric patients aged 6 to 16 years. No relevant differences between the adverse reaction profile for pediatric patients and adult patients were identified. Safety and effectiveness of Prinivil have not been established in pediatric patients under the age of 6 or in pediatric patients with glomerular filtration rate <30 mL/min/1.73 sq m.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Adverse effects reported in greater than 1% of patients receiving lisinopril for the management of heart failure and more frequently than with placebo include dizziness, hypotension, headache, diarrhea, chest pain, nausea, abdominal pain, rash, and upper respiratory tract infection.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2071
For more Drug Warnings (Complete) data for LISINOPRIL (23 total), please visit the HSDB record page.
Lisinopril is indicated for the treatment of acute myocardial infarction, hypertension in patients 6 years, and as an adjunct therapy for heart failure. A combination product with hydrochlorothiazide is indicated for the treatment of hypertension.
FDA Label
Lisinopril is an angiotensin converting enzyme inhibitor used to treat hypertension, heart failure, and myocardial infarction. Lisinopril is not a prodrug, and functions by inhibition of angiotensin converting enzyme as well as the renin angiotensin aldosterone system. It has a wide therapeutic index and a long duration of action as patients are generally given 10-80mg daily.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
C09AA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA03 - Lisinopril
Absorption
Lisinopril is 6-60% orally bioavailable with an average of 25% bioavailability. Lisinopril reaches a Cmax of 58ng/mL with a Tmax of 6-8h. Lisinopril's absorption is not affected by food.
Route of Elimination
Lisinopril is entirely eliminated exclusively in the urine.
Volume of Distribution
The apparent volume of distribution of lisinopril is 124L.
Clearance
A 30kg child has a typical clearance of 10L/h, which increases with renal function. The mean renal clearance of lisinopril in healthy adult males is 121mL/min.
Steady state is attained after two daily doses (every 24 hours) in healthy volunteers. The drug is not metabolized but is eliminated via the kidneys.
Beermann B; Am J Med 85 (3b): 25-30 (1988)
In dogs, lisinopril's bioavilability ranges from 24-50% with peak levels occurring approximately 4 hours after dosing. Lisinopril is distributed poorly into the CNS. It is unknown if it is distributed into maternal milk, but it does cross the placenta.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 857
Following oral administration of Prinivil, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25 percent, with large inter-subject variability (6-60 percent) at all doses tested (5-80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. The absolute bioavailability of lisinopril is reduced to about 16 percent in patients with stable NYHA Class II-IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
For more Absorption, Distribution and Excretion (Complete) data for LISINOPRIL (9 total), please visit the HSDB record page.
Lisinopril is not metabolized and is excreted as the unchanged drug.
Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Lisinopril has an effective half life of accumulation of 12.6h and a terminal half life of 46.7h.
The plasma half-life controlling accumulation during chronic administration is 12-13 hr and the absorbed drug is eliminated via glomerular filtration.
PMID:2550635 Case DE et al; J Hum Hypertens 3 (Suppl 1): 127-31 (1989)
The accumulation half-life averages 12.6 hours despite a terminal serum half-life of approximately 40 hours /in healthy volunteers/.
Beermann B; AmJ Med 85 (3b): 25-30 (1988)
Upon multiple dosing, lisinopril exhibits an effective half-life of 12 hours.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
Angiotensin II constricts coronary blood vessels and is positively inotropic, which under normal circumstances, would increase vascular resistance and oxygen consumption. This action can eventually lead to myocyte hypertrophy and vascular smooth muscle cell proliferation. Lisinopril is an angiotensin converting enzyme inhibitor (ACEI), preventing the conversion of angiotensin I to angiotensin II. This action prevents myocyte hypertrophy and vascular smooth muscle cell proliferation seen in untreated patients. Increased levels of bradykinin also exhibit vasodilating effects for patients taking ACEIs. Lisinopril also inhibits renin's conversion of angiotensin to angiotensin I.
Orally active angiotensin-converting enzyme inhibitor.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1026
Lisinopril inhibits angiotensin converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of lisinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium.
NIH; DailyMed. Current Medication Information for Prinivil (Lisinopril) Tablet (Updated: October 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6f3c339-2c9d-4d07-14a1-6d6c7daf26c6
The action of the angiotensin-converting enzyme (ACE) inhibitor lisinopril on the consequences of myocardial reoxygenation and oxidative damage was assessed in cultured chick embryonic ventricular cardiomyocytes. Lisinopril, 10(-8) M to 10(-6) M, produced a significant (P < 0.05) dose-dependent enhancement of the restoration of contractile frequency occurring during myocardial reoxygenation but did not alter the depression in contractile frequency during hypoxia. Lisinopril significantly (P < 0.05) shifted the dose-response relationship of ammonium persulfate-induced reduction in cardiac contractile frequency. Lisinopril significantly (P < 0.05) reduced the effect of another oxidative agent, tertbutylhydroperoxide which produced a time-dependent reduction in cardiac contractile frequency. Lisinopril did not alter cardiac contractile frequency in the absence of hypoxia or ammonium persulfate or tertbutylhydroperoxide. The viability of cardiomyocytes, assessed by trypan blue exclusion, paralleled the changes in cardiac contractile frequency. Lisinopril significantly (P < 0.05) improved viability of cardiomyocytes exposed to either ammonium persulfate or tertbutylhydroperoxide. Lisinopril did not display any antioxidant properties against the free radical alpha,alpha-diphenyl-beta-picrylhydrazyl. These data suggest that lisinopril accelerates the recovery of cardiomyocytes during reoxygenation and blunts the effects of oxidative agents through mechanisms involving the endogenous renin angiotensin system and/or a direct cellular action.
PMID:8405086 Rabkin; Eur J Pharmacol 238 (1): 81-88 (1993)








