


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
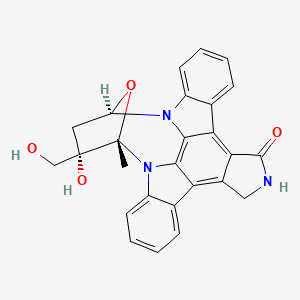

1. Cep 701
2. Cep-701
3. Cep701
4. Kt-555
5. Kt-5555
6. Kt5555
7. Sp-924
8. Sp924
9. Spm-924
1. 111358-88-4
2. Cep-701
3. Kt-5555
4. Spm-924
5. Kt5555
6. Cep 701
7. Sp924
8. Sp 924
9. Sp-924
10. Kt 5555
11. Kt-555
12. Cep701
13. C26h21n3o4
14. Chembl603469
15. Do989gc5d1
16. A-154475.0
17. (9s,10s,12r)-2,3,9,10,11,12-hexahydro-10-hydroxy-10-(hydroxymethyl)-9-methyl-9,12-epoxy-1h-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocin-1-one
18. Ncgc00168772-01
19. (5s,6s,8r)-6-hydroxy-6-(hydroxymethyl)-5-methyl-7,8,14,15-tetrahydro-5h-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacen-13(6h)-one
20. A-154475
21. Lestaurtinib [usan]
22. Lestaurtinib (usan/inn)
23. Lestaurtinib [usan:inn]
24. Unii-do989gc5d1
25. 4otg
26. A 1544750
27. Cep-701 Hydrate
28. Lestaurtinib; Cep701
29. Lestaurtinib [inn]
30. Dsstox_cid_26778
31. Dsstox_rid_81897
32. Dsstox_gsid_46778
33. Lestaurtinib [mart.]
34. Lestaurtinib [who-dd]
35. A 154475.0
36. Gtpl5672
37. Schembl1649693
38. Dtxsid5046778
39. Chebi:91471
40. 9,12-epoxy-1h-diindolo(1,2,3-fg:3',2',1'-kl)pyrrolo(3,4-i)(1,6)benzodiazocin-1-one, 2,3,9,10,11,12-hexahydro-10-hydroxy-10-(hydroxymethyl)-9-methyl-, (9s,10s,12r)-
41. 9,12-epoxy-1h-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocin-1-one, 2,3,9,10,11,12-hexahydro-10-hydroxy-10-(hydroxymethyl)-9-methyl-, (9s,10s,12r)-
42. Bcp06977
43. Zinc3781738
44. Tox21_112633
45. Bdbm50308060
46. Mfcd09840836
47. Nsc772196
48. Nsc800782
49. Akos024457603
50. Ac-5243
51. Am81228
52. Db06469
53. Nsc-772196
54. Nsc-800782
55. Ncgc00168772-02
56. Ncgc00168772-03
57. Ncgc00168772-04
58. Hy-50867
59. Cas-111358-88-4
60. Cs-0004336
61. D04696
62. J-002567
63. Q6531771
64. Brd-k23192422-001-01-1
65. (15s,16s,18r)-16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8,10,12,20,22,24-nonaen-3-one
66. (15s,16s,18r)-16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,14,19-triazaoctacyclo[12.11.2.115,18.02,6.07,27.08,13.019,26.020,25]octacosa-1,6,8(13),9,11,20(25),21,23,26-nonaen-3-one
67. (15s,16s,18r)-16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,14,19-triazaoctacyclo[12.11.2.115,18.02,6.07,27.08,13.019,26.020,25]octacosa-1,6,8,10,12,20,22,24,26-nonaen-3-one
68. (9s,10s,12r)-10-hydroxy-10-(hydroxymethyl)-9-methyl-2,3,9,10,11,12-hexahydro-9,12-epoxy-1h-diindolo(1,2,3-fg:3',2',1'-kl)pyrrolo(3,4-i)(1,6)benzodiazocin-1-one
69. (9s,10s,12r)-2,3,9,10,11,12-hexahyd Ro-10-hydroxy-10-(hydroxymethyl)-9-methyl-9,12-epo Xy-1h-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1, 6]benzodiazocin-1-one
70. 16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8(13),9,11,20(25),21,23-nonaen-3-one
71. 9,12-epoxy-1h-diindolo(1,2,3-fg:3',2',1'-kl)pyrrolo(3,4-i)(1,6)benzodiazocin-1-one, 2,3,9,10,11,12-hexahydro-10-hydroxy-10-(hydroxymethyl)-9-methyl-, (9s-(9alpha,10beta,12alpha))-
| Molecular Weight | 439.5 g/mol |
|---|---|
| Molecular Formula | C26H21N3O4 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 439.15320616 g/mol |
| Monoisotopic Mass | 439.15320616 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 886 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in pancreatic cancer, prostate cancer, and leukemia (myeloid).
Lestaurtinib inhibits autophosphorylation of FMS-like tyrosine kinase 3 (FLT3), resulting in inhibition of FLT3 activity and induction of apoptosis in tumor cells that overexpress FLT3.


