
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
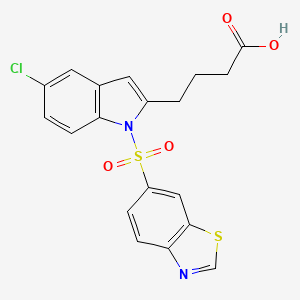

1. Iva337
1. 927961-18-0
2. Iva-337
3. Iva337
4. 28q8ag0pyl
5. Chembl4091374
6. Cpd1537
7. 4-[1-(1,3-benzothiazol-6-ylsulfonyl)-5-chloroindol-2-yl]butanoic Acid
8. 1h-indole-2-butanoic Acid, 1-(6-benzothiazolylsulfonyl)-5-chloro-
9. 5-chloro-1-((6-benzothiazolyl)sulfonyl)-1h-indole-2-butanoic Acid
10. 4-(1-(1,3-benzothiazol-6-ylsulfonyl)-5-chloro-indol-2-yl)butanoic Acid
11. 4-(1-(benzo[d]thiazol-6-ylsulfonyl)-5-chloro-1h-indol-2-yl)butanoic Acid
12. 4-[1-(1,3-benzothiazol-6-ylsulfonyl)-5-chloro-indol-2-yl]butanoic Acid
13. Lanifbranor
14. Libfranor
15. Lanifbranor [inn]
16. Lanifibranor(iva-337)
17. Lanifibranor [inn]
18. Unii-28q8ag0pyl
19. Lanifibranor [who-dd]
20. Schembl3528615
21. Amy16813
22. Cmb96118
23. Ex-a1511
24. Bdbm50244350
25. S8770
26. Akos037649312
27. Db14801
28. Sb18746
29. Ac-31451
30. Bs-17993
31. Hy-104049
32. Cs-0027586
33. A903382
34. Q27896056
35. 1-(6-benzothiazolylsulfonyl)-5-chloro-1h-indole-2-butanoic Acid
36. Bjb
37. Iva-337; Iva 337;4-(1-(1,3-benzothiazol-6-ylsulfonyl)-5-chloro-indol-2-yl)butanoic Acid;iva-337
| Molecular Weight | 434.9 g/mol |
|---|---|
| Molecular Formula | C19H15ClN2O4S2 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 434.0161770 g/mol |
| Monoisotopic Mass | 434.0161770 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 686 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds from the offering will be used to fund the clinical development of Lanifibranor for metabolic dysfunction-associated steatohepatitis (MASH).
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: IVA337
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Leerink Partners | Piper Sandler
Deal Size: $150.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering November 13, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Leerink Partners | Piper Sandler
Deal Size : $150.0 million
Deal Type : Public Offering
Inventiva Announces Pricing of $150M Public Offering of ADS
Details : The proceeds from the offering will be used to fund the clinical development of Lanifibranor for metabolic dysfunction-associated steatohepatitis (MASH).
Product Name : IVA337
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 13, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds from the offering will be used to fund the clinical development of Lanifibranor for metabolic dysfunction-associated steatohepatitis (MASH).
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: IVA337
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Leerink Partners | Piper Sandler
Deal Size: $125.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering November 12, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Leerink Partners | Piper Sandler
Deal Size : $125.0 million
Deal Type : Public Offering
Inventiva Announces Public Offering Launch
Details : The proceeds from the offering will be used to fund the clinical development of Lanifibranor for metabolic dysfunction-associated steatohepatitis (MASH).
Product Name : IVA337
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 12, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The collaboration aims to advance Lanifibranor, a small molecule targeting PPAR-alpha/delta/gamma, to address undisclosed key focus areas.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: IVA337
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Chia Tai Tianqing Pharmaceutical Group
Deal Size: $282.0 million Upfront Cash: $12.0 million
Deal Type: Collaboration July 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Chia Tai Tianqing Pharmaceutical Group
Deal Size : $282.0 million
Deal Type : Collaboration
Inventiva Gets $10M Milestone Payment from CTTQ
Details : The collaboration aims to advance Lanifibranor, a small molecule targeting PPAR-alpha/delta/gamma, to address undisclosed key focus areas.
Product Name : IVA337
Product Type : Miscellaneous
Upfront Cash : $12.0 million
July 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The financing aims to advance the clinical development of company's late stage product IVA337 (lanifibranor), which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: New Enterprise Associates
Deal Size: $233.4 million Upfront Cash: Undisclosed
Deal Type: Financing May 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : New Enterprise Associates
Deal Size : $233.4 million
Deal Type : Financing
Inventiva Secures €116M Second Tranche of €348M Structured Financing
Details : The financing aims to advance the clinical development of company's late stage product IVA337 (lanifibranor), which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
May 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 01, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Inventiva Completes Enrollment in Phase 3 NATiV3 Trial for MASH
Details : IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 01, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Hepalys Pharma
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Hepalys Pharma
Deal Size : Inapplicable
Deal Type : Inapplicable
Inventiva, Hepalys Launch Phase 1 Trial for Lanifibranor in Japan
Details : IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Inventiva Gains Positive DMC Recommendation in MASH Phase 3 Lanifibranor Trial
Details : IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds will fund the completion of the Phase 3 trial and preparation for the potential filing for marketing approval and commercialization of IVA337 (lanifibranor) for adult patients with MASH.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: New Enterprise Associates
Deal Size: $0.3 million Upfront Cash: Undisclosed
Deal Type: Financing October 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : New Enterprise Associates
Deal Size : $0.3 million
Deal Type : Financing
Inventiva Secures €348M for Phase 3 MASH Study NATiV3
Details : The proceeds will fund the completion of the Phase 3 trial and preparation for the potential filing for marketing approval and commercialization of IVA337 (lanifibranor) for adult patients with MASH.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
October 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The Company intends to use the net proceeds to continue the clinical development of lanifibranor , which is being evaluated in the late-stage clinical trial studies for the treatment of MASH.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Samsara BioCapital
Deal Size: $21.8 million Upfront Cash: Undisclosed
Deal Type: Financing July 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Samsara BioCapital
Deal Size : $21.8 million
Deal Type : Financing
Inventiva announces a €20.1 million issuance of royalty certificates
Details : The Company intends to use the net proceeds to continue the clinical development of lanifibranor , which is being evaluated in the late-stage clinical trial studies for the treatment of MASH.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Undisclosed
July 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Lead Product(s): Lanifibranor,Inapplicable
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Lanifibranor,Inapplicable
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Inventiva Announces Positive Lanifibranor DMC Recommendation in NATiV3
Details : IVA-337 (lanifibranor) is a pan-peroxisome proliferator–activated receptor agonist, which is being evaluated for the treatment of Nonalcoholic Steatohepatitis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 16, 2024

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]1,3-benzothiazole-6-sulfonyl chloride
CAS Number : 181124-40-3
End Use API : Lanifibranor
About The Company : LinkChem is a leading China headquartered CMO | CRO provider within the pharmaceutical industry. Our core focus includes: custom synthesis, process development,...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
12
PharmaCompass offers a list of Lanifibranor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lanifibranor manufacturer or Lanifibranor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lanifibranor manufacturer or Lanifibranor supplier.
PharmaCompass also assists you with knowing the Lanifibranor API Price utilized in the formulation of products. Lanifibranor API Price is not always fixed or binding as the Lanifibranor Price is obtained through a variety of data sources. The Lanifibranor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lanifibranor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lanifibranor, including repackagers and relabelers. The FDA regulates Lanifibranor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lanifibranor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Lanifibranor supplier is an individual or a company that provides Lanifibranor active pharmaceutical ingredient (API) or Lanifibranor finished formulations upon request. The Lanifibranor suppliers may include Lanifibranor API manufacturers, exporters, distributors and traders.
Lanifibranor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lanifibranor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lanifibranor GMP manufacturer or Lanifibranor GMP API supplier for your needs.
A Lanifibranor CoA (Certificate of Analysis) is a formal document that attests to Lanifibranor's compliance with Lanifibranor specifications and serves as a tool for batch-level quality control.
Lanifibranor CoA mostly includes findings from lab analyses of a specific batch. For each Lanifibranor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lanifibranor may be tested according to a variety of international standards, such as European Pharmacopoeia (Lanifibranor EP), Lanifibranor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lanifibranor USP).

