
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
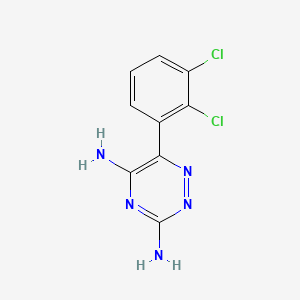

1. 3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine
2. 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine
3. Bw 430c
4. Bw-430c
5. Bw430c
6. Crisomet
7. Labileno
8. Lamictal
9. Lamiktal
1. 84057-84-1
2. 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine
3. Lamictal
4. Lamictal Cd
5. Lamictal Xr
6. Lamotrigina
7. Lamotriginum
8. Bw 430c
9. Lamotriginum [latin]
10. Lamotrigina [spanish]
11. Bw-430c
12. Labileno
13. Lamitor
14. Lamictal Odt
15. 3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine
16. 1,2,4-triazine-3,5-diamine, 6-(2,3-dichlorophenyl)-
17. 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine
18. Bw430c
19. Ltg;bw430c
20. Chembl741
21. Nsc-759171
22. Mls000069685
23. Chebi:6367
24. U3h27498ks
25. Ncgc00015605-06
26. Smr000058464
27. Lamotrigine-13c-d3
28. Dsstox_cid_3195
29. Dsstox_rid_76918
30. Dsstox_gsid_23195
31. Lamictal (tn)
32. Ltg
33. Cas-84057-84-1
34. Sr-01000000187
35. Einecs 281-901-8
36. Mfcd00865333
37. Epilepax
38. Unii-u3h27498ks
39. Hsdb 7526
40. Eur-1048
41. Lamotrigine [usan:usp:inn:ban]
42. Zine-3,5-diamine
43. Lamotrigine- Bio-x
44. Opera_id_12
45. Tocris-1611
46. Hydroxymethyl Progesterone
47. Lamotrigine [mi]
48. Lamotrigine [inn]
49. Lamotrigine [jan]
50. Lopac-l-3791
51. Lamotrigine [hsdb]
52. Lamotrigine [usan]
53. L 3791
54. Lamotrigine [mart.]
55. Lopac0_000688
56. Schembl35439
57. Lamotrigine [usp-rs]
58. Lamotrigine [who-dd]
59. Mls000759486
60. Mls001077325
61. Mls001423991
62. Bidd:gt0794
63. Lamotrigine (jan/usp/inn)
64. Lamotrigine, >=98%, Powder
65. Gtpl2622
66. Dtxsid2023195
67. Zinc13156
68. Lamotrigine [ep Impurity]
69. Lamotrigine [orange Book]
70. Hms2051c10
71. Hms2089m08
72. Hms2093p21
73. Hms2230l04
74. Hms3262i17
75. Hms3268g17
76. Hms3371o16
77. Hms3393c10
78. Hms3657a17
79. Hms3715h21
80. Hms3885m03
81. Lamotrigine [ep Monograph]
82. Lamotrigine [usp Impurity]
83. Pharmakon1600-01505610
84. Lamotrigine [usp Monograph]
85. Amy40805
86. Bcp12156
87. Hy-b0495
88. Lamotrigine 1.0 Mg/ml In Methanol
89. Tox21_110179
90. Tox21_500688
91. Bdbm50031299
92. Nsc746307
93. Nsc759171
94. S3024
95. Stk628377
96. Akos005561147
97. Tox21_110179_1
98. 6-(2,2,4-triazine-3,5-diyldiamine
99. Ccg-100856
100. Db00555
101. Ks-1074
102. Lp00688
103. Nc00106
104. Nsc 746307
105. Nsc 759171
106. Nsc-746307
107. Sdccgsbi-0050666.p003
108. Smp2_000303
109. Ncgc00015605-01
110. Ncgc00015605-02
111. Ncgc00015605-03
112. Ncgc00015605-04
113. Ncgc00015605-05
114. Ncgc00015605-07
115. Ncgc00015605-08
116. Ncgc00015605-09
117. Ncgc00015605-10
118. Ncgc00015605-23
119. Ncgc00015605-24
120. Ncgc00022936-02
121. Ncgc00022936-04
122. Ncgc00022936-05
123. Ncgc00261373-01
124. Ac-10298
125. Ac-32483
126. Bl166799
127. Lamotrigine 100 Microg/ml In Acetonitrile
128. Sbi-0050666.p002
129. 6-(2,3-dichloro-phenyl)-[1,2,4]tria
130. Db-014839
131. B2249
132. Eu-0100688
133. Ft-0602546
134. Ft-0670713
135. Ft-0670714
136. L-205
137. L0241
138. Sw197486-3
139. 57l841
140. A11873
141. D00354
142. W13018
143. Ab00384359-16
144. Ab00384359_17
145. Ab00384359_18
146. A840709
147. Q410346
148. 3,5-diamino-(2,3-dichlorophenyl)-1,2,4-triazine
149. Q-201221
150. Sr-01000000187-2
151. Sr-01000000187-4
152. Sr-01000000187-7
153. Brd-k93460210-071-01-6
154. Sr-01000000187-10
155. 3,5-diamino-6-(2,3,-dichlorophenyl)-1,2,4-triazine
156. 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine.
157. F2173-0540
158. Z1550648755
159. 6-(2,3-dichloro-phenyl)-[1,2,4]triazine-3,5-diamine
160. 6-[2,3-bis(chloranyl)phenyl]-1,2,4-triazine-3,5-diamine
161. Lamotrigine, British Pharmacopoeia (bp) Reference Standard
162. Lamotrigine, European Pharmacopoeia (ep) Reference Standard
163. 1,2,4-triazine-3,5-diamine, 6-(2,3-dichlorophenyl)
164. 6-(2,3-dichloro-phenyl)-[1,2,4]triazine-3,5-diamine(lamotrigine)
165. Gi 267119x; 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine
166. Lamotrigine, United States Pharmacopeia (usp) Reference Standard
167. Lamotrigine, Pharmaceutical Secondary Standard; Certified Reference Material
168. Lamotrigine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
169. Lamotrigine For System Suitability, European Pharmacopoeia (ep) Reference Standard
170. Lamotrigine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 256.09 g/mol |
|---|---|
| Molecular Formula | C9H7Cl2N5 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 255.0078506 g/mol |
| Monoisotopic Mass | 255.0078506 g/mol |
| Topological Polar Surface Area | 90.7 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 242 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Lamictal |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTAL (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lamotri... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 25mg; 150mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 10 | |
|---|---|
| Drug Name | Lamictal cd |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTAL (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lamotri... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 25mg; 5mg; 2mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 10 | |
|---|---|
| Drug Name | Lamictal odt |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTALXR (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lam... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 10 | |
|---|---|
| Drug Name | Lamictal xr |
| Drug Label | Lamotrigine, an antiepileptic drug (AED) of the phenyltriazine class, is chemically unrelated to existing antiepileptic drugs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, extended release |
| Route | oral; Oral |
| Strength | 200mg; 250mg; 25mg; 300mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Smithkline Beecham; Glaxosmithkline |
| 5 of 10 | |
|---|---|
| Drug Name | Lamotrigine |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, extended release; Tablet; Tablet, orally disintegrating; Tablet, chewable |
| Route | oral; Oral |
| Strength | 200mg; 250mg; 25mg; 150mg; 300mg; 5mg; 100mg; 2mg; 50mg |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Anchen Pharms; Teva; Apotex; Alkem Labs; Alembic Pharms; Aurobindo Pharma; Taro; Taro Pharm Inds; Torrent Pharms; Lupin; Sandoz; Cipla; Par Pharm; Hikma Pharms; Watson Labs; Glenmark Generics; Wilshire Pharms; Actavis Elizabeth; Caraco; Jubilan |
| 6 of 10 | |
|---|---|
| Drug Name | Lamictal |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTAL (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lamotri... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 25mg; 150mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 7 of 10 | |
|---|---|
| Drug Name | Lamictal cd |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTAL (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lamotri... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, chewable |
| Route | Oral |
| Strength | 25mg; 5mg; 2mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 8 of 10 | |
|---|---|
| Drug Name | Lamictal odt |
| PubMed Health | Lamotrigine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | LAMICTALXR (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular weight is 256.09. Lam... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 9 of 10 | |
|---|---|
| Drug Name | Lamictal xr |
| Drug Label | Lamotrigine, an antiepileptic drug (AED) of the phenyltriazine class, is chemically unrelated to existing antiepileptic drugs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, its molecular formula is C9H7N5Cl2, and its molecular... |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, extended release |
| Route | oral; Oral |
| Strength | 200mg; 250mg; 25mg; 300mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Smithkline Beecham; Glaxosmithkline |
| 10 of 10 | |
|---|---|
| Drug Name | Lamotrigine |
| Active Ingredient | Lamotrigine |
| Dosage Form | Tablet, extended release; Tablet; Tablet, orally disintegrating; Tablet, chewable |
| Route | oral; Oral |
| Strength | 200mg; 250mg; 25mg; 150mg; 300mg; 5mg; 100mg; 2mg; 50mg |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Anchen Pharms; Teva; Apotex; Alkem Labs; Alembic Pharms; Aurobindo Pharma; Taro; Taro Pharm Inds; Torrent Pharms; Lupin; Sandoz; Cipla; Par Pharm; Hikma Pharms; Watson Labs; Glenmark Generics; Wilshire Pharms; Actavis Elizabeth; Caraco; Jubilan |
Anticonvulsants; Calcium Channel Blockers; Excitatory Amino Acid Antagonists; Voltage-Gated Sodium Channel Blockers
National Library of Medicine's Medical Subject Headings. Lamotrigine. Online file (MeSH, 2015). Available from, as of November 20, 2015: https://www.nlm.nih.gov/mesh/2015/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lamotrigine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of September 30, 2015: https://clinicaltrials.gov/search/intervention=lamotrigine
Lamictal is indicated as adjunctive therapy for the following seizure types in patients aged 2 years and older: partial-onset seizures, primary generalized tonic-clonic (PGTC) seizures, generalized seizures of Lennox-Gastaut syndrome. /Included in the US product label/
NIH; DailyMed. Current Medication Information for Lamictal (Lamotrigine Tablet), Lamictal (Lamotrigine Tablet, Chewable), Lamictal ODT (Lamotrigine Tablet, Orally Disintegrating), Lamictal (Lamotrigine), Lamictal ODT (Lamotrigine) (Updated: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d7e3572d-56fe-4727-2bb4-013ccca22678
Lamcital is indicated for conversion to monotherapy in adults (aged 16 years and older) with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug (AED). /Included in US product label/
NIH; DailyMed. Current Medication Information for Lamictal (Lamotrigine Tablet), Lamictal (Lamotrigine Tablet, Chewable), Lamictal ODT (Lamotrigine Tablet, Orally Disintegrating), Lamictal (Lamotrigine), Lamictal ODT (Lamotrigine) (Updated: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d7e3572d-56fe-4727-2bb4-013ccca22678
For more Therapeutic Uses (Complete) data for LAMOTRIGINE (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: SERIOUS SKIN RASHES. Lamictal can cause serious rashes requiring hospitalization and discontinuation of treatment. The incidence of these rashes, which have included Stevens-Johnson syndrome, is approximately 0.3% to 0.8% in pediatric patients (aged 2 to 17 years) and 0.08% to 0.3% in adults receiving Lamictal. One rash-related death was reported in a prospectively followed cohort of 1,983 pediatric patients (aged 2 to 16 years) with epilepsy taking Lamictal as adjunctive therapy. In worldwide postmarketing experience, rare cases of toxic epidermal necrolysis and/or rash-related death have been reported in adult and pediatric patients, but their numbers are too few to permit a precise estimate of the rate. Other than age, there are as yet no factors identified that are known to predict the risk of occurrence or the severity of rash caused by Lamictal. There are suggestions, yet to be proven, that the risk of rash may also be increased by (1) coadministration of Lamictal with valproate (includes valproic acid and divalproex sodium), (2) exceeding the recommended initial dose of Lamictal, or (3) exceeding the recommended dose escalation for Lamictal. However, cases have occurred in the absence of these factors. Nearly all cases of life-threatening rashes caused by Lamictal have occurred within 2 to 8 weeks of treatment initiation. However, isolated cases have occurred after prolonged treatment (e.g., 6 months). Accordingly, duration of therapy cannot be relied upon as means to predict the potential risk heralded by the first appearance of a rash. Although benign rashes are also caused by Lamictal, it is not possible to predict reliably which rashes will prove to be serious or life threatening. Accordingly, Lamictal should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug related. Discontinuation of treatment may not prevent a rash from becoming life threatening or permanently disabling or disfiguring
NIH; DailyMed. Current Medication Information for Lamictal (Lamotrigine Tablet), Lamictal (Lamotrigine Tablet, Chewable), Lamictal ODT (Lamotrigine Tablet, Orally Disintegrating), Lamictal (Lamotrigine), Lamictal ODT (Lamotrigine) (Updated: May 2015). Available from, as of November 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d7e3572d-56fe-4727-2bb4-013ccca22678
Dizziness, headache, and ataxia were the most frequent adverse nervous system effects, occurring in 38, 29, and 22% of adults, respectively, in controlled trials of lamotrigine adjunctive therapy. The frequency of dizziness and ataxia, and the rate of discontinuance of lamotrigine because of these adverse effects, were dose related in clinical trials; in a dose-response study, dizziness occurred in 54, 31, or 27% of patients receiving lamotrigine 500 mg/day, lamotrigine 300 mg/day, or placebo, respectively, while ataxia occurred in 28, 10, or 10% of those receiving these respective regimens.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2235
Somnolence or insomnia occurred in 14 or 6%, respectively, of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials. Incoordination or tremor was reported in 6 or 4%, respectively, of lamotrigine-treated adults ... Depression occurred in 4%, anxiety in 4%, irritability in 3%, speech disorder in 3%, and concentration disturbance in 2% of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials. Seizure or seizure exacerbation has been reported in 3 or 2% of adults, respectively, receiving lamotrigine as adjunctive therapy in controlled trials; an increase in seizure frequency also has been reported with lamotrigine therapy. Treatment-emergent seizures diagnosed unequivocally as status epilepticus were reported in 7 of 2343 adults receiving adjunctive therapy with lamotrigine in clinical trials; however, the manufacturer states that valid estimates of the incidence of treatment-emergent status epilepticus are difficult to obtain because of variations in the definitions used by different investigators to identify such cases.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2235
Coordination abnormality, dizziness, anxiety, and insomnia occurred in 7, 7, 5, and 5% respectively, of adults receiving lamotrigine as monotherapy in a controlled trial; amnesia, ataxia, asthenia, depression, hypesthesia, libido increase, decreased or increased reflexes, nystagmus, and irritability each occurred in 2% of such patients. Paresthesia or asthenia occurred in more than 1% of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials but with equal or greater frequency in those receiving placebo.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2235
For more Drug Warnings (Complete) data for LAMOTRIGINE (42 total), please visit the HSDB record page.
Lamotrigine is indicated as adjunctive therapy for the following seizure types in patients 2 years of age: partial seizures, primary generalized tonic-clonic seizures, and generalized seizures due to Lennox-Gastaut syndrome. It is also indicated for the process of conversion to drug monotherapy for those at least 16 years of age or older with partial seizures and currently are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug (AED). In addition to the above, lamotrigine is also indicated for the maintenance treatment of bipolar I disorder, delaying the time to mood episodes (which may include mania, hypomania, depression, mixed episodes) in adults at least 18 years or older, who have been treated for acute mood symptoms with standard therapy. Limitations of use It is important to note that lamotirigine should not be used in the treatment of acute mood episodes, as efficacy has not been established in this context.
FDA Label
Lamotrigine likely prevents seizures and prevents mood symptoms via stabilizing presynaptic neuronal membranes and preventing the release of excitatory neurotransmitters such as glutamate, which contribute to seizure activity. A note on cardiovascular effects The metabolite of lamotrigine, 2-N-methyl metabolite (formed by glucuronidation), is reported to cause dose-dependent prolongations of the PR interval, widening of the QRS complex, and at higher doses, complete AV block. Although this harmful metabolite is only found in trace amounts in humans, plasma concentrations may increase in conditions that cause decreased drug glucuronidation, such as liver disease.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)
N03AX09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX09 - Lamotrigine
Absorption
Lamotrigine is rapidly and entirely absorbed with minimal first-pass metabolism effects, with a bioavailability estimated at 98%. Cmax is reached in the range of 1.4 to 4.8 hours post-dose, but this depends on the dose administered, concomitant medications, and epileptic status. The rate and extent of lamictal absorption is considered equivalent between the compressed tablet form taken with water to that of the chewable dispersible tablets, taken with or without water.
Route of Elimination
Lamotrigine is excreted in both the urine and feces. Following oral administration of 240 mg radiolabelled lamotrigine, about 94% of total drug and its metabolites administered is recovered in the urine and 2% is recovered in the feces. One pharmacokinetic study recovered 43 to 87% of a lamotrigine dose in the urine mainly as glucuronidated metabolites. 2-N-glucuronide is mainly excreted in the urine.
Volume of Distribution
The mean apparent volume of distribution (Vd/F) of lamotrigine following oral administration ranges from 0.9 to 1.3 L/kg and is independent of dose administered. Lamotrigine accumulated in the kidney of the male rat, and likely behaves in a similar fashion in humans. Lamotrigine also binds to tissues containing melanin, such as the eyes and pigmented skin.
Clearance
The mean apparent plasma clearance (Cl/F) ranges from 0.18 to 1.21 mL/min/kg. The values vary depending on dosing regimen, concomitant antiepileptic medications, and disease state of the individual. In one study, healthy volunteers on lamictal monotherapy showed a clearance of about 0.44 mL/min/kg after a single dose.
/MILK/ Lamotrigine is distributed into milk. Because of the potential for serious adverse reactions to lamotrigine in nursing infants, a decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2239
/MILK/ To investigate the pharmacokinetics of lamotrigine (LTG) during delivery, during the neonatal period, and lactation. High-performance liquid chromatography was used to determine plasma and milk levels of LTG in nine pregnant women with epilepsy treated with LTG, and plasma levels in their 10 infants. Samples were obtained at delivery, the first 3 days postpartum, and at breast-feeding 2-3 weeks after delivery. At delivery, maternal plasma LTG concentrations were similar to those from the umbilical cord, indicating extensive placental transfer of LTG. There was a slow decline in the LTG plasma concentration in the newborn. At 72 hr postpartum, median LTG plasma levels in the infants were 75% of the cord plasma levels (range, 50-100%). The median milk/maternal plasma concentration ratio was 0.61 (range, 0.47-0.77) 2-3 weeks after delivery, and the nursed infants maintained LTG plasma concentrations of approximately 30% (median, range 23-50%) of the mother's plasma levels. Maternal plasma LTG concentrations increased significantly during the first 2 weeks after parturition, the median increase in plasma concentration/dose ratio being 170%.
PMID:10840403 Ohman I et al; Epilepsia 41 (6): 709-13 (2000)
Lamotrigine binds to melanin-containing ocular tissue in pigmented rats and cynomolgus monkeys, but evidence of this manifestation has not been reported in humans ... prolonged administration of the drug could potentially result in its accumulation and possible toxic effects in melanin-rich tissues, including those of the eye, and that clinicians should be aware of possible adverse ophthalmologic effects occurring as a result of binding of the drug to melanin.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2238
To determine the relative bioavailability of lamotrigine (LTG) chewable dispersible tablets after rectal administration. Two-period, crossover study with a 2-week washout between dosing periods. Twelve healthy adult volunteers. One hundred milligrams of a LTG chewable dispersible tablet was administered by oral and rectal routes. Plasma samples were collected before and up to 120 hours after drug administration. The samples were analyzed for LTG by high-performance liquid chromatography, and the relative bioavailability was determined. Drug concentrations were lower after rectal than after oral administration. The relative bioavailability (F = AUC(rectal)/AUC(oral)) was 0.52 +/- 0.23 (SD). Drug prepared from LTG chewable dispersible tablets is absorbed rectally, although not to the same extent as when given orally. Rectal administration of suspension of these tablets can be an acceptable route of administration.
PMID:11213851 Birnbaum A et al; Pharmacotherapy 21 (2): 158-62 (2001)
For more Absorption, Distribution and Excretion (Complete) data for LAMOTRIGINE (9 total), please visit the HSDB record page.
Lamotrigine is mainly glucuronidated, forming 2-N-glucuronide conjugate, a pharmacologically inactive metabolite. The total radioactivity detected after a 240mg radiolabeled dose of lamotrigine during clinical trials were as follows: lamotrigine as unchanged drug(10%), a 2-N-glucuronide (76%), a 5-N-glucuronide (10%), a 2-N-methyl metabolite (0.14%), as well as various other minor metabolites (4%).
The metabolites of [(14)C]lamotrigine (78 micromol/kg, iv) in adult male Wistar rats were characterized with particular reference to thioether derivatives of an epoxide intermediate. Biliary recovery of radioactivity from anesthetized and cannulated animals was 7.3 +/- 3.0% (mean +/- SD, n = 4) of the dose over 4 hr; 5.5 +/- 0.5% was recovered in bladder urine after 4 hr. Bile contained [(14)C]lamotrigine (1.4 +/- 0.3%), a glutathione adduct of [(14)C]dihydrohydroxylamotrigine (1.8 +/- 0.3%), i.e., an adduct of an arene oxide, and the glutathione (1.5 +/- 0.7%), cysteinylglycine (1.9 +/- 0.5%), and N-acetylcysteine (0.4 +/- 0.2%) adducts of [(14)C]lamotrigine. Formation of the thioether metabolites was partially blocked by the cytochrome P450 inhibitor, ketoconazole. Urine contained [(14)C]lamotrigine (4.5 +/- 0.5%) and [(14)C]lamotrigine N-oxide (0.9 +/- 0.2%). The radiolabeled material in skin (15.6 +/- 1.4%) was almost entirely [(14)C]lamotrigine. ...
PMID:11087428 Maggs J et al; Chem Res Toxicol 13 (11): 1075-81 (2000)
The average elimination half-life of lamotrigine ranges from approximately 14-59 hours. The value is dependent on the dose administered, concomitant drug therapy, as well as disease status. One pharmacokinetic study revealed a half-life of 22.8 to 37.4 hours in healthy volunteers. It also reported that enzyme-inducing antiepileptic drugs such as pheobarbital, phenytoin, or carbamazepine decrease the half-life of lamotrigine. On the other hand, valproic acid increases the half-life of lamotrigine (in the range of 48-59 hours).
/Investigators/ describe the findings in a patient following the deliberate ingestion of a large amount of lamotrigine (stated 4.5 g, absorbed estimated 2.9 g) ... Peak measured concentration of lamotrigine was 35.8 mg/L and half-life 19.5 hours, suggesting linear kinetics in overdose.
PMID:11185974 O'Donnell J et al; J Toxicol Clin Toxicol 38 (6): 659-60 (2000)
The plasma half-life of a single dose is 24 to 35 hr. Administration of phenytoin, carbamazepine, phenobarbital, or primidone reduces the half-life of lamotrigine to approximately 15 hr and reduces plasma concentrations of lamotrigine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 539
The exact mechanism of action of lamotrigine is not fully elucidated, as it may exert cellular activities that contribute to its efficacy in a range of conditions. Although chemically unrelated, lamotrigine actions resemble those of phenytoin and carbamazepine, inhibiting voltage-sensitive sodium channels, stabilizing neuronal membranes, thereby modulating the release of presynaptic excitatory neurotransmitters. Lamotrigine likely acts by inhibiting sodium currents by selective binding to the inactive sodium channel, suppressing the release of the excitatory amino acid, glutamate. The mechanism of action of lamotrigine in reducing anticonvulsant activity is likely the same in managing bipolar disorder. Studies on lamotrigine have identified its binding to sodium channels in a fashion similar to local anesthetics, which could explain the demonstrated clinical benefit of lamotrigine in some neuropathic pain states. Lamotrigine displays binding properties to several different receptors. In laboratory binding assays, it demonstrates weak inhibitory effect on the serotonin 5-HT3 receptor. Lamotrigine also weakly binds to Adenosine A1/A2 receptors, 1/2/ adrenergic receptors, dopamine D1/D2 receptors, GABA A/B receptors, histamine H1 receptors, -opioid receptor (KOR), mACh receptors and serotonin 5-HT2 receptors with an IC50>100 M. Weak inhibitory effects were observed at sigma opioid receptors. An in vivo study revealed evidence that lamotrigine inhibits Cav2.3 (R-type) calcium currents, which may also contribute to its anticonvulsant effects.
Spectrophotometry with the Ca(++)-sensitive dye fura-2 was used to study the effect of lamotrigine (LAG) on the depolarization-evoked Ca++ influx in the acutely isolated basolateral amygdala neurons. Depolarization of the neurons with high K+ resulted in the elevation of intracellular Ca++ concentration [Ca++]i in a concentration-dependent manner. The K(+)-induced Ca++ influx was completely blocked in the Ca(++)-free solution or by Cd++, indicating that depolarization-induced increases in [Ca++]i were triggered largely, if not completely, by Ca++ entry from extracellular space and Ca++ entry occurred through voltage-dependent Ca++ channels. Application of LAG reduced the depolarization-evoked Ca++ influx in a concentration-dependent manner. The effect of LAG was markedly reduced in the presence of N-type Ca++ channel blocker omega-conotoxin-GVIA (omega-CgTX). These results suggest that the action of LAG is mediated, at least in part, by the modulation of N-type Ca++ channels.
PMID:9661253 Wang S et al; Synapse 29 (4): 355-62 (1998)
Lamotrigine (LAG) is an antiepileptic drug which is believed to suppress seizures by inhibiting the release of excitatory neurotransmitters. The present study was aimed at investigating the effect of LAG on the 4-aminopyridine (4AP)-evoked glutamate release in cerebrocortical nerve terminals (synaptosomes). LAG inhibited the release of glutamate evoked by 4AP in a concentration-dependent manner. This inhibitory effect was associated with a reduction in the depolarization-evoked increase in the cytoplasmic free Ca2+ concentration ([Ca2+]C). In addition, LAG did not alter the resting synaptosomal membrane potential or 4AP-evoked depolarization. Furthermore, ionomycin-evoked glutamate release was not affected by LAG. Based on these results, we suggest that presynaptic calcium influx blockade and inhibition of glutamate release may underlie the mechanism of action of LAG. These action may also contribute to their neuroprotective properties in excitotoxic injury.
PMID:11447345 Wang S et al; Neuroreport 12 (10): 2255-8 (2001)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
37
PharmaCompass offers a list of Lamotrigine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lamotrigine manufacturer or Lamotrigine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lamotrigine manufacturer or Lamotrigine supplier.
PharmaCompass also assists you with knowing the Lamotrigine API Price utilized in the formulation of products. Lamotrigine API Price is not always fixed or binding as the Lamotrigine Price is obtained through a variety of data sources. The Lamotrigine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lamictal manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lamictal, including repackagers and relabelers. The FDA regulates Lamictal manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lamictal API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lamictal manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lamictal supplier is an individual or a company that provides Lamictal active pharmaceutical ingredient (API) or Lamictal finished formulations upon request. The Lamictal suppliers may include Lamictal API manufacturers, exporters, distributors and traders.
click here to find a list of Lamictal suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lamictal DMF (Drug Master File) is a document detailing the whole manufacturing process of Lamictal active pharmaceutical ingredient (API) in detail. Different forms of Lamictal DMFs exist exist since differing nations have different regulations, such as Lamictal USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lamictal DMF submitted to regulatory agencies in the US is known as a USDMF. Lamictal USDMF includes data on Lamictal's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lamictal USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lamictal suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lamictal Drug Master File in Japan (Lamictal JDMF) empowers Lamictal API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lamictal JDMF during the approval evaluation for pharmaceutical products. At the time of Lamictal JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lamictal suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lamictal Drug Master File in Korea (Lamictal KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lamictal. The MFDS reviews the Lamictal KDMF as part of the drug registration process and uses the information provided in the Lamictal KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lamictal KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lamictal API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lamictal suppliers with KDMF on PharmaCompass.
A Lamictal CEP of the European Pharmacopoeia monograph is often referred to as a Lamictal Certificate of Suitability (COS). The purpose of a Lamictal CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lamictal EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lamictal to their clients by showing that a Lamictal CEP has been issued for it. The manufacturer submits a Lamictal CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lamictal CEP holder for the record. Additionally, the data presented in the Lamictal CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lamictal DMF.
A Lamictal CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lamictal CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lamictal suppliers with CEP (COS) on PharmaCompass.
A Lamictal written confirmation (Lamictal WC) is an official document issued by a regulatory agency to a Lamictal manufacturer, verifying that the manufacturing facility of a Lamictal active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lamictal APIs or Lamictal finished pharmaceutical products to another nation, regulatory agencies frequently require a Lamictal WC (written confirmation) as part of the regulatory process.
click here to find a list of Lamictal suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lamictal as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lamictal API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lamictal as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lamictal and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lamictal NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lamictal suppliers with NDC on PharmaCompass.
Lamictal Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lamictal GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lamictal GMP manufacturer or Lamictal GMP API supplier for your needs.
A Lamictal CoA (Certificate of Analysis) is a formal document that attests to Lamictal's compliance with Lamictal specifications and serves as a tool for batch-level quality control.
Lamictal CoA mostly includes findings from lab analyses of a specific batch. For each Lamictal CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lamictal may be tested according to a variety of international standards, such as European Pharmacopoeia (Lamictal EP), Lamictal JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lamictal USP).

