


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
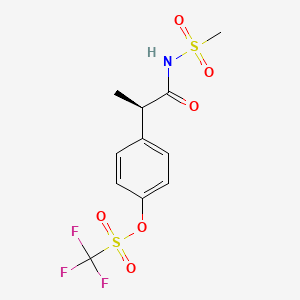

1. 2'-((4'-trifluoromethanesulfonyloxy)phenyl)-n-methanesulfonylpropionamide
2. Df 2156a
3. Df 2156y
4. Df-2156a
5. Df-2156y
6. Df2156a
7. Df2156y
1. 849776-05-2
2. Ladarixin [inn]
3. (r)-4-[1-(methylsulfonamido)-1-oxo-2-propyl]phenyl Trifluoromethanesulfonate
4. 4-((2r)-1-oxo-1-(methanesulfonamido)propan-2-yl)phenyl Trifluoromethanesulfonate
5. Deh7q6472o
6. 849776-05-2 (free)
7. Methanesulfonic Acid, 1,1,1-trifluoro-, 4-((1r)-1-methyl-2-((methylsulfonyl)amino)-2-oxoethyl)phenyl Ester
8. Methanesulfonic Acid, Trifluoro-, 4-((1r)-1-methyl-2-((methylsulfonyl)amino)-2-oxoethyl)phenyl Ester
9. Df2156a
10. Unii-deh7q6472o
11. Ladarixin [who-dd]
12. Schembl251618
13. Chembl189475
14. Dtxsid50234030
15. Df-2156a
16. Ac6925
17. Bdbm50475370
18. Akos037649625
19. Cs-12792
20. Hy-19519
21. Cs-0015618
22. A857904
23. Q27276351
24. (r)-(-)-n-(2-(4-(trifluoromethanesulfonyloxy)phenyl)propionyl)methanesulfonamide
25. [4-[(2r)-1-(methanesulfonamido)-1-oxopropan-2-yl]phenyl] Trifluoromethanesulfonate
| Molecular Weight | 375.3 g/mol |
|---|---|
| Molecular Formula | C11H12F3NO6S2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 375.00581394 g/mol |
| Monoisotopic Mass | 375.00581394 g/mol |
| Topological Polar Surface Area | 123 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 624 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of type I diabetes mellitus


