
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
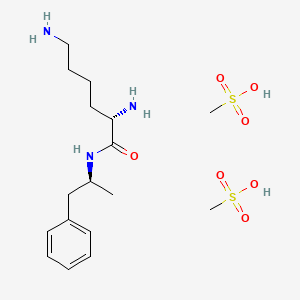

1. Dimesylate, Lis-dexamfetamine
2. Dimesylate, Lisdexamfetamine
3. Elvanse
4. Lis Dexamfetamine Dimesylate
5. Lis-dexamfetamine Dimesylate
6. Lisdexamfetamine
7. Nrp 104
8. Nrp-104
9. Nrp104
10. Vyvanse
1. Vyvanse
2. 608137-33-3
3. Nrp-104
4. Lisdexamfetamine Mesilate
5. Spd489
6. Tyvense
7. Nrp104
8. Spd-489
9. Lisdexamfetamine Dimesylate [usan]
10. Lisdexamfetamine Dimesilate
11. Sjt761gegs
12. Lisdexamphetamine Dimesilate
13. L-lysine-d-amphetamine Dimesylate
14. Lisdexamfetamine Dimethanesulfonate
15. (2s)-2,6-diamino-n-[(1s)-1-methyl-2-phenylethyl]hexanamide Dimethanesulfonate
16. Lis-dexamfetamine Dimesylate
17. Lisdexamfetamine Mesilate (jan)
18. Lisdexamfetamine Dimesylate (usan)
19. Lisdexamfetamine Mesylate
20. Nrp 104
21. Lisdexamfetamine Mesilate [jan]
22. (2s)-2,6-diamino-n-[(2s)-1-phenylpropan-2-yl]hexanamide;methanesulfonic Acid
23. Unii-sjt761gegs
24. Venvanse
25. (2s)-2,6-diamino-n-((1s)-1-methyl-2-phenylethyl)hexanamide Dimethanesulfonate
26. Lys-amp
27. Spd 489
28. Lys-d-amp
29. Elvanse (tn)
30. Vyvanse (tn)
31. Schembl678421
32. Chembl1201178
33. Dtxsid60209653
34. Lisdexamfetamine Dimesylate Solution
35. Bcp24044
36. Akos030254940
37. Lisdexamfetamine Mesilate [mart.]
38. Ldx
39. Lisdexamfetamine Mesilate [who-dd]
40. Lisdexamfetamine Dimesylate [vandf]
41. D04747
42. Lisdexamfetamine Dimethanesulfonate [mi]
43. Lisdexamfetamine Dimesylate [orange Book]
44. 137l333
45. Q27289243
46. (2s)-2,6-diamino-n-((1s)-1-methyl-2-phenylethyl)hexanamide Dimethanesulphonate
47. Hexanamide, 2,6-diamino-n-((1s)-1-methyl-2-phenylethyl), (2s), Dimethanesulfonate
48. Hexanamide, 2,6-diamino-n-((1s)-1-methyl-2-phenylethyl), (2s), Dimethanesulphonate
49. Ldx;lisdexamfetamine Mesilate;lisdexamfetamine Mesylate;nrp 104;nrp-104;spd 489
50. Lisdexamfetamine Dimesylate Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 455.6 g/mol |
|---|---|
| Molecular Formula | C17H33N3O7S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 455.17599275 g/mol |
| Monoisotopic Mass | 455.17599275 g/mol |
| Topological Polar Surface Area | 207 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 344 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Lisdexamfetamine dimesylate |
| Drug Label | Vyvanse (lisdexamfetamine dimesylate), a CNS stimulant,isa capsule for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate.... |
| Active Ingredient | Lisdexamfetamine dimesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 40mg; 70mg; 20mg |
| Market Status | Tentative Approval |
| Company | Mylan Pharms; Amneal Pharms; Roxane |
| 2 of 4 | |
|---|---|
| Drug Name | Vyvanse |
| PubMed Health | Amphetamine (By mouth) |
| Drug Classes | Amphetamine |
| Drug Label | Vyvanse (lisdexamfetamine dimesylate), a CNS stimulant,isa capsule for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate.... |
| Active Ingredient | Lisdexamfetamine dimesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 40mg; 20mg; 70mg |
| Market Status | Prescription |
| Company | Shire Development |
| 3 of 4 | |
|---|---|
| Drug Name | Lisdexamfetamine dimesylate |
| Drug Label | Vyvanse (lisdexamfetamine dimesylate), a CNS stimulant,isa capsule for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate.... |
| Active Ingredient | Lisdexamfetamine dimesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 40mg; 70mg; 20mg |
| Market Status | Tentative Approval |
| Company | Mylan Pharms; Amneal Pharms; Roxane |
| 4 of 4 | |
|---|---|
| Drug Name | Vyvanse |
| PubMed Health | Amphetamine (By mouth) |
| Drug Classes | Amphetamine |
| Drug Label | Vyvanse (lisdexamfetamine dimesylate), a CNS stimulant,isa capsule for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate.... |
| Active Ingredient | Lisdexamfetamine dimesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 50mg; 60mg; 40mg; 20mg; 70mg |
| Market Status | Prescription |
| Company | Shire Development |
Treatment of Attention Deficit Hyperactivity Disorder (ADHD)
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Dopamine Uptake Inhibitors
Drugs that block the transport of DOPAMINE into axon terminals or into storage vesicles within terminals. Most of the ADRENERGIC UPTAKE INHIBITORS also inhibit dopamine uptake. (See all compounds classified as Dopamine Uptake Inhibitors.)
GDUFA
DMF Review : Complete
Rev. Date : 2023-11-21
Pay. Date : 2023-10-12
DMF Number : 38874
Submission : 2023-10-20
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2020-01-28
Pay. Date : 2020-01-21
DMF Number : 22442
Submission : 2009-01-27
Status : Active
Type : II
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
GDUFA
DMF Review : Complete
Rev. Date : 2021-12-30
Pay. Date : 2021-11-18
DMF Number : 35645
Submission : 2021-11-23
Status : Active
Type : II
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 42282
Submission : 2025-07-11
Status : Active
Type : II
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36325
Submission : 2021-10-25
Status : Active
Type : II

USDMF
GDUFA
DMF Review : Complete
Rev. Date : 2025-04-21
Pay. Date : 2025-04-08
DMF Number : 38667
Submission : 2023-08-03
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20103
Submission : 2006-12-29
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-07-02
Pay. Date : 2018-05-22
DMF Number : 32289
Submission : 2018-05-18
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2020-07-28
Pay. Date : 2020-06-03
DMF Number : 34823
Submission : 2020-05-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32210
Submission : 2017-11-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Veranova is a global leader in developing and manufacturing specialist and complex APIs for pharma and biotech customers, with over 50 years of experience supporting the healthcare...
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
About the Company : Noramco, founded in 1979, specializes in developing and manufacturing APIs for opioid and non-opioid products. It excels in controlled substance development, offering supply-chain ...
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
About the Company : Supriya Lifescience Limited, established in 1987 and headquartered in Mumbai, is a globally recognized, technology-driven manufacturer of APIs, CDMO, and formulations. Its faciliti...
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
About the Company : Cohance Lifesciences is a leading CDMO and API platform delivering products and services across the full molecule lifecycle, from development to commercialization. With strong expe...
About the Company : Temad Co., established in 1997, is one of Iran's largest API producers and an innovative manufacturer of narcotic and non-narcotic products in the Middle East. It complies with Ira...
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
About the Company : Malladi Drugs & Pharmaceuticals Ltd, founded in 1980 by Mr. M L N Sastry, is a leader in manufacturing ephedrine and pseudoephedrine salts. It is now the top API manufacturer in th...
About the Company : Since 1962, MOEHS has produced high-quality Active Pharmaceutical Ingredients (APIs) for the global market. With decades of technical expertise, Moehs Group delivers pharmaceutical...
 Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
Siegfried – A global CDMO delivering integrated pharmaceutical development and manufacturing solutions.
About the Company : Siegfried is a global Contract Development and Manufacturing Organization providing integrated services for pharmaceutical ingredients & finished dosage forms. With 13 production s...
About the Company : Anvitha Life Care Private Limited, founded in 2016 by Dr. T. Prakasam and a team of experienced scientists and technocrats with over 80 years of combined expertise, is located on a...

About the Company : Established in 1991, SM Biomed is a joint venture company pioneering the manufacture of API (active pharmaceutical ingredients) products in Malaysia. Company has grown several fold...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2040-04-16
US Patent Number : 12433859
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 219847
Patent Use Code : U-842
Delist Requested :
Patent Use Description : INDICATED FOR THE TREA...
Patent Expiration Date : 2040-04-16

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
41
PharmaCompass offers a list of Lisdexamfetamine Dimesylate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lisdexamfetamine Dimesylate manufacturer or Lisdexamfetamine Dimesylate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lisdexamfetamine Dimesylate manufacturer or Lisdexamfetamine Dimesylate supplier.
PharmaCompass also assists you with knowing the Lisdexamfetamine Dimesylate API Price utilized in the formulation of products. Lisdexamfetamine Dimesylate API Price is not always fixed or binding as the Lisdexamfetamine Dimesylate Price is obtained through a variety of data sources. The Lisdexamfetamine Dimesylate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L-Lysine-d-amphetamine dimesylate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L-Lysine-d-amphetamine dimesylate, including repackagers and relabelers. The FDA regulates L-Lysine-d-amphetamine dimesylate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L-Lysine-d-amphetamine dimesylate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L-Lysine-d-amphetamine dimesylate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L-Lysine-d-amphetamine dimesylate supplier is an individual or a company that provides L-Lysine-d-amphetamine dimesylate active pharmaceutical ingredient (API) or L-Lysine-d-amphetamine dimesylate finished formulations upon request. The L-Lysine-d-amphetamine dimesylate suppliers may include L-Lysine-d-amphetamine dimesylate API manufacturers, exporters, distributors and traders.
click here to find a list of L-Lysine-d-amphetamine dimesylate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L-Lysine-d-amphetamine dimesylate DMF (Drug Master File) is a document detailing the whole manufacturing process of L-Lysine-d-amphetamine dimesylate active pharmaceutical ingredient (API) in detail. Different forms of L-Lysine-d-amphetamine dimesylate DMFs exist exist since differing nations have different regulations, such as L-Lysine-d-amphetamine dimesylate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L-Lysine-d-amphetamine dimesylate DMF submitted to regulatory agencies in the US is known as a USDMF. L-Lysine-d-amphetamine dimesylate USDMF includes data on L-Lysine-d-amphetamine dimesylate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L-Lysine-d-amphetamine dimesylate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L-Lysine-d-amphetamine dimesylate suppliers with USDMF on PharmaCompass.
A L-Lysine-d-amphetamine dimesylate written confirmation (L-Lysine-d-amphetamine dimesylate WC) is an official document issued by a regulatory agency to a L-Lysine-d-amphetamine dimesylate manufacturer, verifying that the manufacturing facility of a L-Lysine-d-amphetamine dimesylate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting L-Lysine-d-amphetamine dimesylate APIs or L-Lysine-d-amphetamine dimesylate finished pharmaceutical products to another nation, regulatory agencies frequently require a L-Lysine-d-amphetamine dimesylate WC (written confirmation) as part of the regulatory process.
click here to find a list of L-Lysine-d-amphetamine dimesylate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing L-Lysine-d-amphetamine dimesylate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for L-Lysine-d-amphetamine dimesylate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture L-Lysine-d-amphetamine dimesylate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain L-Lysine-d-amphetamine dimesylate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a L-Lysine-d-amphetamine dimesylate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of L-Lysine-d-amphetamine dimesylate suppliers with NDC on PharmaCompass.
L-Lysine-d-amphetamine dimesylate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L-Lysine-d-amphetamine dimesylate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L-Lysine-d-amphetamine dimesylate GMP manufacturer or L-Lysine-d-amphetamine dimesylate GMP API supplier for your needs.
A L-Lysine-d-amphetamine dimesylate CoA (Certificate of Analysis) is a formal document that attests to L-Lysine-d-amphetamine dimesylate's compliance with L-Lysine-d-amphetamine dimesylate specifications and serves as a tool for batch-level quality control.
L-Lysine-d-amphetamine dimesylate CoA mostly includes findings from lab analyses of a specific batch. For each L-Lysine-d-amphetamine dimesylate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L-Lysine-d-amphetamine dimesylate may be tested according to a variety of international standards, such as European Pharmacopoeia (L-Lysine-d-amphetamine dimesylate EP), L-Lysine-d-amphetamine dimesylate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L-Lysine-d-amphetamine dimesylate USP).

