
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
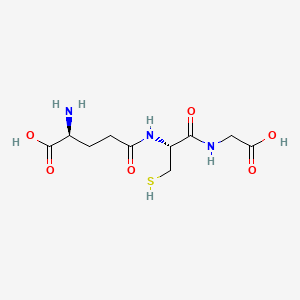

1. Gamma L Glu L Cys Gly
2. Gamma L Glutamyl L Cysteinylglycine
3. Gamma-l-glu-l-cys-gly
4. Gamma-l-glutamyl-l-cysteinylglycine
5. Glutathione, Reduced
6. Reduced Glutathione
1. 70-18-8
2. L-glutathione
3. Glutathion
4. Isethion
5. L-glutathione Reduced
6. Tathion
7. Reduced Glutathione
8. Glutathione-sh
9. Glutinal
10. Tathione
11. Deltathione
12. Neuthion
13. Copren
14. Glutide
15. Triptide
16. Ledac
17. Gsh
18. Glutatione
19. Glutatiol
20. Glutathione Reduced
21. Panaron
22. Glutathione Sh
23. L-glutatione
24. Agifutol S
25. Glutathione (reduced)
26. L-gamma-glutamyl-l-cysteinylglycine
27. Gamma-l-glutamyl-l-cysteinylglycine
28. Glutathione [jan]
29. L-glutathione, Reduced
30. Gamma-l-glutamyl-l-cysteinyl-glycine
31. 5-l-glutamyl-l-cysteinylglycine
32. Glutham
33. Gamma-l-glutamylcysteinylglycine
34. Glutathione Red
35. Aec Glutathione
36. Red. Glutathione
37. Bakezyme Rx
38. L-glutathione Reduce
39. Glycine, L-gamma-glutamyl-l-cysteinyl-
40. Reduced L-glutathione
41. (2s)-2-amino-5-[[(2r)-1-(carboxymethylamino)-1-oxo-3-sulfanylpropan-2-yl]amino]-5-oxopentanoic Acid
42. (s)-2-amino-5-(((r)-1-((carboxymethyl)amino)-3-mercapto-1-oxopropan-2-yl)amino)-5-oxopentanoic Acid
43. N-(n-gamma-l-glutamyl-l-cysteinyl)glycine
44. N-(n-l-gamma-glutamyl-l-cysteinyl)glycine
45. Glycine, N-(n-l-gamma-glutamyl-l-cysteinyl)-
46. Gan16c9b8o
47. L-glutamyl-l-cysteinylglycine
48. L-glutathione (reduced Form)
49. Glycine, L-.gamma.-glutamyl-l-cysteinyl-
50. Benzenamine, 2-[(4-methoxyphenyl)methoxy]-
51. Chebi:16856
52. (2s)-2-amino-4-{[(1r)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic Acid
53. 106272-20-2
54. 95687-20-0
55. Dsstox_cid_3101
56. N-(n-l-.gamma.-glutamyl-l-cysteinyl)glycine
57. N5-((r)-1-((carboxymethyl)amino)-3-mercapto-1-oxopropan-2-yl)-l-glutamine
58. Dsstox_rid_76875
59. Dsstox_gsid_23101
60. [glu(-cys)]n-gly
61. Mfcd00065939
62. Cas-70-18-8
63. (s)-2-amino-5-((r)-1-(carboxymethylamino)-3-mercapto-1-oxopropan-2-ylamino)-5-oxopentanoic Acid
64. Glutathione, Reduced
65. Ccris 2094
66. Sr-05000002567
67. Glutathione [ban:jan]
68. Einecs 200-725-4
69. L-glutathione Reduced Form
70. Unii-gan16c9b8o
71. Nsc 400639
72. Phytochelatins
73. Readisorb
74. Glutathione;
75. Nsc400639
76. 1lbk
77. Ncgc00094976-01
78. Tathion (tn)
79. Glutathione (jp17)
80. Spectrum_000419
81. 1oe7
82. 1oe8
83. 1r4w
84. C(n-.gamma.glu-)g
85. Glutathione [ii]
86. Glutathione [mi]
87. Reduced Glutathione,(s)
88. Spectrum2_001500
89. Spectrum3_000946
90. Spectrum4_001056
91. Spectrum5_000940
92. Glutathione, Reduced Form
93. Glutathione [inci]
94. Bmse000185
95. Bmse000952
96. Bmse000956
97. Glutathione (reduced Type)
98. Glutathione [vandf]
99. Cys(n-.gamma.glu-)-gly
100. Schembl9167
101. Chembl1543
102. Glutathione [mart.]
103. Glutathione [usp-rs]
104. Glutathione [who-dd]
105. Kbiogr_001352
106. Kbioss_000899
107. Mls001333069
108. Divk1c_000075
109. Spectrum1502248
110. Spbio_001519
111. Gamma-glutamyl-cysteinyl-glycine
112. Gtpl6737
113. Dtxsid6023101
114. L-?-glutamyl-l-cysteinylglycine
115. L-glutathione Reduced, 97.0%
116. Chebi:60836
117. Hms500d17
118. Kbio1_000075
119. Kbio2_000899
120. Kbio2_003467
121. Kbio2_006035
122. Kbio3_002012
123. (gamma-glutamylcysteine)n-glycine
124. L-g-glutamyl-l-cysteinyl-glycine
125. Y-l-glutamyl-l-cysteinyl-glycine
126. L-?-glutamyl-l-cysteinyl-glycine
127. Ninds_000075
128. Glutathione [ep Monograph]
129. Hms1921n22
130. Pharmakon1600-01502248
131. Poly(gamma-glutamylcysteine)glycine
132. Hy-d0187
133. L-glutathione Reduced, >=98.0%
134. Zinc3830891
135. Gam.-l-glutamyl-l-cysteinyl-glycine
136. Tox21_111371
137. Bdbm50422268
138. Ccg-38876
139. L-gamma-glutamyl-l-cysteinyl-glycine
140. Nsc758199
141. S4606
142. Akos015999135
143. Tox21_111371_1
144. Cs-7948
145. Db00143
146. Nsc-758199
147. Sdccgmls-0066687.p001
148. .gamma.-l-glutamyl-l-cysteinyl-glycine
149. Idi1_000075
150. N-(n-l-?-glutamyl-l-cysteinyl)glycine
151. Pharm Biol 11: 539 (1968)
152. Smp1_000247
153. Ncgc00264046-02
154. Ds-14675
155. Gsh;gamma-l-glutamyl-l-cysteinyl-glycine
156. Smr000857220
157. Sbi-0051743.p002
158. L-glutathione Reduced, Bioxtra, >=98.0%
159. B7775
160. G0074
161. Glycine, N-(n-l-gamma-glutamyl-l-cysteinyl)
162. C00051
163. C02471
164. D00014
165. G-3980
166. P19615
167. Ab00443568_03
168. Glutathione 100 Microg/ml In Acetonitrile:water
169. A866658
170. Q116907
171. Sr-05000002567-1
172. Sr-05000002567-2
173. L-glutathione Reduced, Vetec(tm) Reagent Grade, >=98%
174. Z2183947556
175. Glutathione, European Pharmacopoeia (ep) Reference Standard
176. Glutathione, United States Pharmacopeia (usp) Reference Standard
177. Glutathione, Pharmaceutical Secondary Standard; Certified Reference Material
178. L-glutathione Reduced, Cell Culture Tested, Bioreagent, >=98.0%, Powder
179. (2s)-2-amino-4-{[(1r)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoylbutanoic Acid
180. (2s)-2-amino-5-({(2r)-1-[(carboxymethyl)amino]-1-oxo-3-sulfanyl-2-propanyl}amino)-5-oxopentanoic Acid
181. Glutathione; L-glutathione Reduced; 5-l-glutamyl-l-cysteinylglycine; Gamma-l-glutamyl-l-cysteinylglycine; Gsh
| Molecular Weight | 307.33 g/mol |
|---|---|
| Molecular Formula | C10H17N3O6S |
| XLogP3 | -4.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 307.08380644 g/mol |
| Monoisotopic Mass | 307.08380644 g/mol |
| Topological Polar Surface Area | 160 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 389 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For nutritional supplementation, also for treating dietary shortage or imbalance
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB32 - Glutathione
Absorption
Research suggests that glutathione is not orally bioactive, and that very little of oral glutathione tablets or capsules is actually absorbed by the body.
Glutathione (GSH) participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase. It also plays a role in the hepatic biotransformation and detoxification process; it acts as a hydrophilic molecule that is added to other lipophilic toxins or wastes prior to entering biliary excretion. It participates in the detoxification of methylglyoxal, a toxic by-product of metabolism, mediated by glyoxalase enzymes. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. GSH is a cofactor of conjugation and reduction reactions that are catalyzed by glutathione S-transferase enzymes expressed in the cytosol, microsomes, and mitochondria. However, it is capable of participating in non-enzymatic conjugation with some chemicals, as it is hypothesized to do to a significant extent with n-acetyl-p-benzoquinone imine (NAPQI), the reactive cytochrome P450 reactive metabolite formed by toxic overdose of acetaminophen. Glutathione in this capacity binds to NAPQI as a suicide substrate and in the process detoxifies it, taking the place of cellular protein sulfhydryl groups which would otherwise be toxically adducted. The preferred medical treatment to an overdose of this nature, whose efficacy has been consistently supported in literature, is the administration (usually in atomized form) of N-acetylcysteine, which is used by cells to replace spent GSSG and allow a usable GSH pool.
 Bioquim: An European GMP-certified company manufacturing bulk APIs, specializing in sterile lyophilization and chemical synthesis.
Bioquim: An European GMP-certified company manufacturing bulk APIs, specializing in sterile lyophilization and chemical synthesis.
About the Company : Bioquim is a European company specializing in bulk oral and sterile APIs for the pharmaceutical industry. With over 40 years of experience, the company supplies corticosteroids, pe...
About the Company : As an ISO9001, ISO22000 certificated supplier, Basic Nutrition supply high-quality ingredients for nutraceuticals. We can offer a range of products from small to large quantites: h...
 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
About the Company : Willow Birch Pharma, Inc. is a premier supplier of bulk APIs to North American Compounding Pharmacies, sourcing from FDA registered and GMP manufacturers. With licenses in all 50 s...
About the Company : Sumar Biotech LLP, registered in January 2018, is a startup approved by the Gujarat State Biotechnology Mission. Founded by young entrepreneurs and experienced research and manufac...
 Anlon Healthcare manufactures high-quality Pharmaceutical Bulk Drugs & Intermediates compliant with FDA, PMDA, KFDA, cGMP & WHO-GMP.
Anlon Healthcare manufactures high-quality Pharmaceutical Bulk Drugs & Intermediates compliant with FDA, PMDA, KFDA, cGMP & WHO-GMP.
About the Company : Anlon Healthcare is a research-focused pharmaceutical manufacturer based in Rajkot, specializing in bulk drugs and intermediates. The company’s products comply with international...
About the Company : Chongqing Daxin was established in 1941. It is a core member of PKUCare pharmaceutical industry. After nearly 70 year's development, it has become a biotech oriented and comprehens...

About the Company : Gurvey & Berry offers an impressive lineup of bulk raw materials, but we are much more than products by the kilo or tonne. With 4 decades behind us, and our Service First philosoph...

About the Company : Established in 2010, Mac-Chem is a NMM Group company. Mac-Chem is focused on oncology and other niche specialty APIs. It supplies quality APIs to the top 15 oncology players in Ind...

About the Company : Shenzhen GSH Bio-tech Co., Ltd is a professional manufacture focusing on research, development and producing the reduced glutathione by biocatalytic method. The annual production c...

About the Company : Summit ingredient Co.,Ltd is a leading and rapidly growing company wh ich engages in the production, development, source and sale of botanical extracts . Summit located in Shaanxi ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
53
PharmaCompass offers a list of Glutathione API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Glutathione manufacturer or Glutathione supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Glutathione manufacturer or Glutathione supplier.
PharmaCompass also assists you with knowing the Glutathione API Price utilized in the formulation of products. Glutathione API Price is not always fixed or binding as the Glutathione Price is obtained through a variety of data sources. The Glutathione Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L-Glutathione manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L-Glutathione, including repackagers and relabelers. The FDA regulates L-Glutathione manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L-Glutathione API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L-Glutathione manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L-Glutathione supplier is an individual or a company that provides L-Glutathione active pharmaceutical ingredient (API) or L-Glutathione finished formulations upon request. The L-Glutathione suppliers may include L-Glutathione API manufacturers, exporters, distributors and traders.
click here to find a list of L-Glutathione suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L-Glutathione DMF (Drug Master File) is a document detailing the whole manufacturing process of L-Glutathione active pharmaceutical ingredient (API) in detail. Different forms of L-Glutathione DMFs exist exist since differing nations have different regulations, such as L-Glutathione USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L-Glutathione DMF submitted to regulatory agencies in the US is known as a USDMF. L-Glutathione USDMF includes data on L-Glutathione's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L-Glutathione USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L-Glutathione suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The L-Glutathione Drug Master File in Japan (L-Glutathione JDMF) empowers L-Glutathione API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the L-Glutathione JDMF during the approval evaluation for pharmaceutical products. At the time of L-Glutathione JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of L-Glutathione suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a L-Glutathione Drug Master File in Korea (L-Glutathione KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of L-Glutathione. The MFDS reviews the L-Glutathione KDMF as part of the drug registration process and uses the information provided in the L-Glutathione KDMF to evaluate the safety and efficacy of the drug.
After submitting a L-Glutathione KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their L-Glutathione API can apply through the Korea Drug Master File (KDMF).
click here to find a list of L-Glutathione suppliers with KDMF on PharmaCompass.
A L-Glutathione CEP of the European Pharmacopoeia monograph is often referred to as a L-Glutathione Certificate of Suitability (COS). The purpose of a L-Glutathione CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of L-Glutathione EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of L-Glutathione to their clients by showing that a L-Glutathione CEP has been issued for it. The manufacturer submits a L-Glutathione CEP (COS) as part of the market authorization procedure, and it takes on the role of a L-Glutathione CEP holder for the record. Additionally, the data presented in the L-Glutathione CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the L-Glutathione DMF.
A L-Glutathione CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. L-Glutathione CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of L-Glutathione suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing L-Glutathione as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for L-Glutathione API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture L-Glutathione as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain L-Glutathione and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a L-Glutathione NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of L-Glutathione suppliers with NDC on PharmaCompass.
L-Glutathione Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L-Glutathione GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L-Glutathione GMP manufacturer or L-Glutathione GMP API supplier for your needs.
A L-Glutathione CoA (Certificate of Analysis) is a formal document that attests to L-Glutathione's compliance with L-Glutathione specifications and serves as a tool for batch-level quality control.
L-Glutathione CoA mostly includes findings from lab analyses of a specific batch. For each L-Glutathione CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L-Glutathione may be tested according to a variety of international standards, such as European Pharmacopoeia (L-Glutathione EP), L-Glutathione JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L-Glutathione USP).

