


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
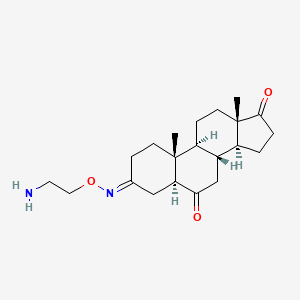

1. 3-((2-aminoethoxy)imino)androstane-6,17-dione
2. 3-((2-aminoethoxy)imino)androstane-6,17-dione Hydrochloride
3. Pst 2744
4. Pst-2744
5. Pst2744
1. 203737-93-3
2. Istaroxime, (e)-
3. Pst-2744
4. W40687go3s
5. (3e,5s,8r,9s,10r,13s,14s)-3-(2-aminoethoxyimino)-10,13-dimethyl-1,2,4,5,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthrene-6,17-dione
6. 203738-46-9
7. 3-((2-aminoethoxy)imino)-5alpha-androstan-6,17-dione
8. Debio-0614
9. Istaroxime [inn]
10. Unii-w8i9h2tppl
11. Pst 2744
12. St-2744
13. W8i9h2tppl
14. Unii-w40687go3s
15. Chembl469045
16. Schembl7285904
17. Dtxsid20870222
18. Akos024259258
19. Cs-1531
20. Db06157
21. Ac-36855
22. As-78198
23. Hy-15718
24. 5-alpha-androstan-6,17-dione, 3-((2-aminoethoxy)imino)-
25. Androstane-3,6,17-trione, 3-(o-(2-aminoethyl)oxime), (3e,5alpha)-
26. Androstane-3,6,17-trione, 3-(o-(2-aminoethyl)oxime), (3e,5.alpha.)-
| Molecular Weight | 360.5 g/mol |
|---|---|
| Molecular Formula | C21H32N2O3 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 360.24129289 g/mol |
| Monoisotopic Mass | 360.24129289 g/mol |
| Topological Polar Surface Area | 81.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in heart disease.
Reduces the sodium-potassium adenosine triphosphatase (ATPase) activity and stimulates the sarcoplasmic calcium ATPase isoform 2 reuptake function.


