



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
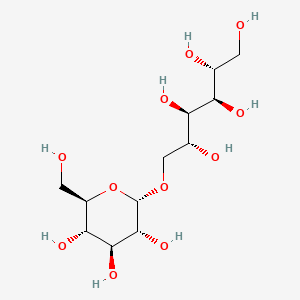

1. D-glucitol, 6-o-alpha-d-glucopyranosyl-, Mixt. With 1-o-alpha-d-glucopyranosyl-d-mannitol
2. D-glucitol, 6-o-alpha-d-glucopyranosyl-, Mixture With 1-o-alpha-d-glucopyranosyl-d-mannitol
3. Palatinit
1. 20942-99-8
2. 1-o-alpha-d-glucopyranosyl-d-mannitol
3. Palatinit
4. 64519-82-0
5. D-mannitol, 1-o-.alpha.-d-glucopyranosyl-
6. (2r,3r,4r,5r)-6-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexane-1,2,3,4,5-pentaol
7. 1,1-gpm
8. G97p6s66e9
9. Palatinitol
10. (2r,3r,4r,5r)-6-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,4,5-pentol
11. Isomaltidex
12. Wurcs=2.0/2,2,1/[h1122h][a2122h-1a_1-5]/1-2/a6-b1
13. Galeniq 980
14. A-d-glcp-1,1-d-mannitol
15. 1-o-d-glucopyranosyl-d-mannitol
16. Glucosylmannitol
17. Unii-s870p55o2w
18. Ccris 3698
19. Hsdb 7969
20. Einecs 244-122-4
21. Dsstox_cid_753
22. Dsstox_rid_75770
23. Dsstox_gsid_20753
24. Glucosylmannitol [mi]
25. Schembl154031
26. Unii-g97p6s66e9
27. Chembl3187473
28. Dtxsid60872321
29. Chebi:150326
30. Chebi:192265
31. S870p55o2w
32. (3r,4r,5r)-6-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)hexane-1,2,3,4,5-pentaol
33. 6-o-a-d-glucopyranosyl-d-glucitol
34. 6-o-a-d-glucopyranosyl-d-mannitol
35. Gpm
36. Tox21_200914
37. Mfcd03414194
38. Zinc31319586
39. 6-o-alpha-d-glucopyranosyl-d-glucitol Mixed With 1-o-alpha-d-glucopyranosyl-d-mannitol
40. Ncgc00248874-01
41. Ncgc00258468-01
42. 1-o-.alpha.-d-glucopyranosyl-d-mannitol
43. D-mannitol, 1-o-alpha-d-glucopyranosyl-
44. Cas-64519-82-0
45. 1-o-(a-glucopyranosyl)-d-mannitol Dihydrate
46. A14329
47. 6-o-.alpha.-d-glucopyranosyl-d-mannitol
48. A914114
49. A919358
50. Q412068
51. W-109182
52. 570fc73a-80f5-464f-b47e-b9b4cc544121
53. D-glucitol, 6-o-alpha-d-glucopyranosyl-, Mixed With 1-o-alpha-d-glucopyranosyl-d-mannitol
54. D-glucitol, 6-o-alpha-d-glucopyranosyl-, Mixt. With 1-o-alpha-d-glucopyranosyl-d-mannitol
55. (2r,3r,4r,5r)-6-((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yloxy)hexane-1,2,3,4,5-pentaol
| Molecular Weight | 344.31 g/mol |
|---|---|
| Molecular Formula | C12H24O11 |
| XLogP3 | -5.2 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 344.13186158 g/mol |
| Monoisotopic Mass | 344.13186158 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Disaccharides; Sugar Alcohols; Sweetening Agents; Cariogenic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
By means of programmed feeding experiments on rats, the caries-producing properties of a new sugar substitute Palatinit were compared with those of other carbohydrates. The cariogenicity of Palatinit was significantly lower than that of sucrose and lactose and roughly comparable to that of L-sorbose. Reference strains of Streptococcus mutans are unable to produce extra-cellular polysaccharide or notable amounts of acid from Palatinit. The use of this substance as sugar substitute can be recommended for caries prophylaxis on the basis of these experiments.
PMID:274266 Karle EJ, Gehring E; Dtsch Zahnarztl Z 33 (3): 189-91 (1978)
Cariogenic Agents
Substances that promote DENTAL CARIES. (See all compounds classified as Cariogenic Agents.)
The in vivo metabolism of isomalt in the large intestine was simulated in an in vitro fermentation study to investigate its degradation using chyme from pigs as a basic substrate additionally inoculated with feces. In the first week, the fermentation of isomalt (3.65%) by non-adapted microflora was investigated. In the second week, isomalt fermentation by adapted microflora taken from pigs fed a basic diet supplemented with isomalt was studied. In the third week, both /non-adapted and adapted/ flora were studied in fermentation experiments with a high concentration of isomalt (7.30%). Isomalt was degraded to lactic acid, volatile fatty acids, and gases (CO2, CH4, and hydrogen). ...
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
Fistulated and normal pigs were fed 10% sucrose between meals, 5 or 10% isomalt between meals, or 10% isomalt with meals. The passage and absorption rate of these substances were determined at the terminal ileum (10 pigs per treatment) or over the whole distance of the digestive tract (4 pigs per treatment). Ten percent sucrose was completely digested and absorbed in the small intestine. In the 3 isomalt treatments, 61-64% of the ingested compound passed the terminal ileum in the form of intact isomalt plus free sorbitol, free mannitol, and free glucose. None of these sugars were excreted in the feces, indicating that isomalt and its constituents passing the terminal ileum are completely broken down in the large intestine.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
Renal clearance studies were conducted in adult female rats (250 g b.w.) infused with 1.8 g isomalt, alpha-O-D-glucopyranosyl-1,6-D-sorbitol, or alpha-O-D-glucopyranosyl-1,6-D-mannitol over a period of 3 hours. Maximum plasma concentrations of 25 mM were obtained. These compounds were readily cleared and urinary concentrations of up to 100 mg/mL were recorded, which compares with a maximum urinary concentration of 0.6 mg/mL in rats receiving 5 g isomalt per day orally. After the infusion of either isomalt or alpha-O-D-glucopyranosyl-1,6-D-sorbitol, free sorbitol was not detected in blood or urine, and blood glucose concentrations were unchanged, demonstrating the metabolic inertness of these disaccharide alcohols. From the infusion and excretion rates and the plasma concentrations that were observed, the authors concluded that alpha-O-D-glucopyranosyl-1,6-D-sorbitol is distributed in extracellular water, but does not reach the intracellular compartments.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
When isomalt was fed to rats for several weeks it was observed that fecal excretion declined steadily, while the cecum enlarged. The authors concluded that this resulted from adaptation and metabolism by the gut microflora. Similarly, during a 17-day feeding period in which 6 female rats received 3.5 g isomalt daily, the fecal content fell from 25% of the dose at the beginning to 1% at the end.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for Isomalt (9 total), please visit the HSDB record page.
Rat intestinal maltase was shown to be active against isomalt, alpha-O-D-glucopyranosyl-1,6-D-sorbitol, and alpha-O-D-glucopyranosyl-1,6-D-mannitol, but the rates of hydrolysis were slow. The ratio of the rates of hydrolysis of sucrose, isomaltulose, and isomalt by rat intestinal alpha-glucosidases was 100:30:12. Similarly, sucrose was hydrolysed about 20 times faster than alpha-O-D-glucopyranosyl-1,6-D-sorbitol or alpha-O-D-glucopyranosyl-1,6-D-mannitol by disaccharidases from the small intestine of the pig, and the relative rates of hydrolysis of maltose, sucrose, isomaltulose and isomalt by human intestinal alpha-glucosidases were 100:25:11:2.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
The fate of isomalt in the gastrointestinal tract of female rats that had been adapted to the compound was investigated by increasing its dietary concentration from 10% to 34.5% over a period of 3-4 weeks. After administration of 1.7 g isomalt in 5 g feed, the contents of the stomach, small intestine, cecum, and large intestine were examined at intervals up to 6 hr. From the content of alpha-O-D-glucopyranosyl-1,6-D-sorbitol, alpha-O-D-glucopyranosyl-1,6-D-mannitol, sorbitol, mannitol, and sucrose found in these organs, the authors concluded that alpha-O-D-glucopyranosyl-1,6-D-sorbitol and alpha-O-D-glucopyranosyl-1,6-D-mannitol were only partially hydrolyzed by the carbohydrases in the small intestine, while a substantial proportion of these compounds reached the cecum where further hydrolysis of glycosidic bonds occurred. Fermentation of the liberated hexitols occurred in the cecum, which was enlarged, and only small amounts of alpha-O-D-glucopyranosyl-1,6-D-sorbitol, alpha-O-D-glucopyranosyl-1,6-D-mannitol, and hexitols reached the large intestine.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 20: Isomalt (64519-82-0) (1985). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
/Investigators/ compared the effect of a variety of sugar alcohols on calcium absorption from the rat small and large intestine in vitro. An Using chamber technique was used to determine the net transport of Ca across the epithelium isolated from the jejunum, ileum, cecum, and colon of rats. The concentration of Ca in the serosal and mucosal Tris buffer solution was 1.25 mM and 10 mM, respectively. The Ca concentration in the serosal medium was determined after incubation for 30 min and the net Ca absorption was evaluated. The addition of 0.1-200 mM erythritol, xylitol, sorbitol, maltitol, palatinit, or lactitol to the mucosal medium affected net Ca absorption in the intestinal preparations. Differences in Ca transport were observed between portions of the intestine, but not between sugar alcohols tested. /The authors/ concluded that sugar alcohols directly affect the epithelial tissue and promote Ca absorption from the small and large intestine in vitro.
PMID:12064809 Mineo H et al; Dig Dis Sci 47 (6): 1326-33 (2002)
Isomalt is a non-cariogenic sweetener, which is widely used in sugar-free candy and chewing gum. Little is known about the effects of Isomalt on de- and remineralization. Binding between calcium and Isomalt has been reported, which could affect the mineral balance. The objective of this study was to examine the effects of Isomalt on de- and remineralization of bovine enamel lesions, both in vitro and in situ. In in vitro study, subsurface enamel lesions were subjected to 3-weeks pH-cycling. Treatments were 5-min rinses with 10% Isomalt solutions daily and 10% Isomalt additions to re- or demineralizing solutions. Standard pH-cycling conditions were used with a 0.2 ppm fluoride background during the remineralization phase. In in situ study, subsurface lesions were exposed 2 months in vivo and brushed three times daily with 10% Isomalt containing toothpaste. Treatment effects were assessed by chemical analysis of the solutions (in vitro) and transversal microradiography (in vitro and in situ). In in vitro study, while 5-min rinses with 10% Isomalt gave slightly increased remineralization, continuous presence of 10% Isomalt (in re- or demineralizing solutions) inhibited both de- and/or remineralization. This lead to significantly smaller overall mineral loss when Isomalt was added during demineralization. In in situ study, remineralization enhancement during short Isomalt treatments was confirmed. Isomalt had a positive effect on the de/remineralization balance when given under conditions relevant to practical use.
PMID:18157558 Takatsuka T et al; Clin Oral Investig 12 (2): 173-7 (2008)
... Reports from authoritative bodies and reviews indicates that the decrease in pH in plaque as a consequence of metabolic acid production by saccharolytic bacteria when exposed to fermentable carbohydrates (i.e. sugars and starches) may promote demineralization and prevent remineralization of the hydroxyapatite crystals. Tooth hydroxyapatite crystals are very resistant to dissolution at neutral pH, but their solubility drastically increases as pH drops. Typically, the critical pH for dental enamel is around 5.5. ... Demineralization of tooth tissues can also occur as a result of consumption of dietary acids in foods or beverages, and that frequent consumption can lead to dental erosion. Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose are slowly metabolized by bacteria in the mouth. The rate and amount of acid production from these food constituents is significantly less than that from sucrose. ... Xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose do not promote dental caries because they do not lower plaque pH to the level associated with enamel demineralization. ... A cause and effect relationship has been established between the consumption of sugar-containing foods/drinks at an exposure frequency of four times daily or more and an increased tooth demineralization, and that the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose, instead of sugar in sugar-containing foods/drinks, may maintain tooth mineralization by decreasing tooth demineralization compared with sugar-containing foods, provided that such foods/drinks do not lead to dental erosion.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm
The food constituents xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose resulted in reduced post-prandial blood glucose (or insulinemic) responses compared with sugars on a weight by weight basis owing to their reduced/delayed digestion/absorption and/or to a decrease in the amount of available carbohydrates, and that the consumption of foods/drinks in which xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose replaced sugars induced lower post-prandial glycemic and insulinemic responses than sugar-containing foods/drinks. ... A cause and effect relationship has been established between the consumption of foods/drinks containing xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose or polydextrose instead of sugar and reduction in post-prandial blood glucose responses (without disproportionally increasing post-prandial insulinemic responses) as compared to sugar-containing foods/drinks.
European Food Safety Authority (EFSA); EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation and reduction of post-prandial glycaemic responses (April 2011). Available from, as of July 28, 2011: https://www.efsa.europa.eu/en/publications.htm


