
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
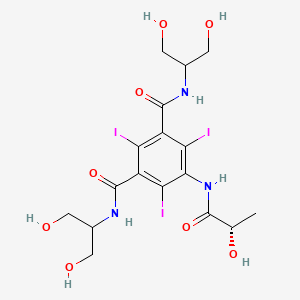

1. B 15,000
2. B 15000
3. B-15,000
4. B-15000
5. B15,000
6. B15000
7. Gastromiro
8. Iopamidol, (+-)-isomer
9. Iopamidol, (r)-isomer
10. Iopamidol, Sodium Salt, (s)-isomer
11. Iopamiro
12. Isovue
13. Isovue 370
14. Jopamidol
15. Niopam
16. Solutrast
17. Solutrast 370
18. Solutrast Gastro
19. Sq 13,396
1. 60166-93-0
2. Iopamiron
3. Isovue
4. Iopamiro
5. Niopam
6. Solutrast
7. Iomapidol
8. Gastromiro
9. Iopamidolum
10. B-15000
11. Sq-13396
12. Sq 13,396
13. 62883-00-5
14. (s)-n,n'-bis(2-hydroxy-1-(hydroxymethyl)ethyl)-2,4,6-triiodo-5-lactamidoisophthalamide
15. L-(+)-n,n'-bis(2-hydroxy-1-hydroxymethylethyl)-2,4,6-triiodo-5-lactamide Isophthalamide
16. L-5alpha-hydroxypropionylamino-2,4,6-triiodoisophthalic Acid Di(1,3-dihydroxy-2-propylamide)
17. Chebi:31711
18. Jr13w81h44
19. (s)-n1,n3-bis(1,3-dihydroxypropan-2-yl)-5-(2-hydroxypropanamido)-2,4,6-triiodoisophthalamide
20. Iopamidol 300
21. Solutrast 370
22. Ncgc00016892-01
23. Oypalomin
24. Isovue 370
25. Cas-60166-93-0
26. Dsstox_cid_3158
27. Dsstox_rid_76896
28. Dsstox_gsid_23158
29. 1,3-benzenedicarboxamide, N,n'-bis(2-hydroxy-1-(hydroxymethyl)ethyl)-5-((2-hydroxy-1-oxopropyl)amino)-2,4,6-triiodo-, (s)-
30. 1-n,3-n-bis(1,3-dihydroxypropan-2-yl)-5-[[(2s)-2-hydroxypropanoyl]amino]-2,4,6-triiodobenzene-1,3-dicarboxamide
31. Iopamiron 300
32. Iopamiron 370
33. Jopamiron 200
34. Iopamiro 370
35. Isovue-370
36. Niopam 300
37. Iopamidolum [inn-latin]
38. N,n'-bis(1,3-dihydroxypropan-2-yl)-5-{[(2s)-2-hydroxypropanoyl]amino}-2,4,6-triiodobenzene-1,3-dicarboxamide
39. Iopamidol-200
40. Iopamidol-250
41. Iopamidol-300
42. Iopamidol-370
43. Scanlux-300
44. Scanlux-370
45. Isovue-128
46. Isovue-200
47. Isovue-250
48. Isovue-300
49. Isovue-m 200
50. Isovue-m 300
51. Einecs 262-093-6
52. B 15000
53. Brn 6250226
54. Iopamyron
55. Sq 13396
56. Unii-jr13w81h44
57. Hsdb 8075
58. Iopamiron (tn)
59. Iopamidol-200 In Plastic Container
60. Iopamidol-250 In Plastic Container
61. Iopamidol-300 In Plastic Container
62. Iopamidol-370 In Plastic Container
63. Isovue (tn)
64. Iopamidol [usan:usp:inn:ban:jan]
65. Iopamidol [inn]
66. Iopamidol [jan]
67. Iopamidol [mi]
68. Iopamidol [usan]
69. Prestwick0_000871
70. Prestwick1_000871
71. Prestwick2_000871
72. Prestwick3_000871
73. Iopamidol [vandf]
74. Iopamidol (jp17/usp)
75. Iopamidol [mart.]
76. Iopamidol [who-dd]
77. L-5alpha-idrossipropionilamino-2,4,6-triiodoisoftal-di(1,3-diidrossi-2-propilamide)
78. Schembl27781
79. Bspbio_000941
80. Spbio_002862
81. Bpbio1_001037
82. Iopamidol [ep Impurity]
83. Iopamidol [orange Book]
84. Chembl1200932
85. Dtxsid1023158
86. Iopamidol [ep Monograph]
87. Iopamidol [usp Monograph]
88. Hms1570p03
89. Hms2097p03
90. Hms3714p03
91. Pharmakon1600-01502304
92. Act03261
93. Hy-b0684
94. Zinc3830947
95. Tox21_110668
96. Nsc759636
97. S4532
98. Akos015891034
99. Tox21_110668_1
100. Ccg-213024
101. Db08947
102. Ks-1421
103. Ncgc00016892-02
104. Ncgc00016892-04
105. (s)-n,n Inverted Exclamation Marka-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide
106. (s)-n,n'-bis(2-hydroxy-1-(hydroxymethyl)ethyl)-5-((2-hydroxy-1-oxopropyl)amino)-2,4,6-triiodoisophthaldiamide
107. 1,3-benzenedicarboxamide, N,n'-bis(2-hydroxy-1-(hydroxymethyl)ethyl)-5-(((2s)-2-hydroxy-1-oxopropyl)amino)-2,4,6-triiodo-
108. N1,n3-bis(1,3-dihydroxypropan-2-yl)-5-(2-hydroxypropanoylamino)-2,4,6-triiodo-benzene-1,3-dicarboxamide
109. Ab00513941
110. D01797
111. H11976
112. Hydroxypropanamido)-2,4,6-triiodoisophthalamide
113. Ab00513941_02
114. 166i930
115. A834067
116. Q424788
117. Q-201245
118. (s)-n1,n3-bis(1,3-dihydroxypropan-2-yl)-5-(2-
119. Brd-k75868704-001-01-2
120. (s)-n,n'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxopropyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide
121. N,n'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2s)-2-hydroxy-1-oxopropyl]amino]-2,4,6-triiodo-1,3-benzenedicarboxamide
122. N,n'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-{[(2s)-2-hydroxypropanoyl]amino}-2,4,6-triiodobenzene-1,3-dicarboxamide
123. N1,n3-bis(1,3-dihydroxypropan-2-yl)-5-[(2s)-2-hydroxypropanamido]-2,4,6-triiodobenzene-1,3-dicarboxamide
| Molecular Weight | 777.1 g/mol |
|---|---|
| Molecular Formula | C17H22I3N3O8 |
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 776.8541 g/mol |
| Monoisotopic Mass | 776.8541 g/mol |
| Topological Polar Surface Area | 188 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 22 | |
|---|---|
| Drug Name | Iopamidol-250 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 22 | |
|---|---|
| Drug Name | Iopamidol-300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 3 of 22 | |
|---|---|
| Drug Name | Iopamidol-370 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 4 of 22 | |
|---|---|
| Drug Name | Isovue-200 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 41% |
| Market Status | Prescription |
| Company | Bracco |
| 5 of 22 | |
|---|---|
| Drug Name | Isovue-250 |
| Drug Label | Diagnostic NONIONIC RADIOPAQUE CONTRAST MEDIAFor Intrathecal Administration in NeuroradiologyIncluding Myelography (Lumbar, Thoracic, Cervical, Total Columnar) Pediatric Myelography (Lumbar, Thoracic), and for Contrast Enhancement of Computed Tomogra... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Bracco |
| 6 of 22 | |
|---|---|
| Drug Name | Isovue-300 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Bracco |
| 7 of 22 | |
|---|---|
| Drug Name | Isovue-370 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Bracco |
| 8 of 22 | |
|---|---|
| Drug Name | Isovue-m 200 |
| Drug Label | ISOVUE (lopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration.Each mL of ISOVUE-200 (lopamidol Injection 41%) provides 408 mg iopamidol with 1 mg tromethamine and 0.26 mg edetate c... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 41% |
| Market Status | Prescription |
| Company | Bracco |
| 9 of 22 | |
|---|---|
| Drug Name | Isovue-m 300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Bracco |
| 10 of 22 | |
|---|---|
| Drug Name | Scanlux-300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Sanochemia Corp Usa |
| 11 of 22 | |
|---|---|
| Drug Name | Scanlux-370 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Sanochemia Corp Usa |
| 12 of 22 | |
|---|---|
| Drug Name | Iopamidol-250 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 13 of 22 | |
|---|---|
| Drug Name | Iopamidol-300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 14 of 22 | |
|---|---|
| Drug Name | Iopamidol-370 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 15 of 22 | |
|---|---|
| Drug Name | Isovue-200 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 41% |
| Market Status | Prescription |
| Company | Bracco |
| 16 of 22 | |
|---|---|
| Drug Name | Isovue-250 |
| Drug Label | Diagnostic NONIONIC RADIOPAQUE CONTRAST MEDIAFor Intrathecal Administration in NeuroradiologyIncluding Myelography (Lumbar, Thoracic, Cervical, Total Columnar) Pediatric Myelography (Lumbar, Thoracic), and for Contrast Enhancement of Computed Tomogra... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Bracco |
| 17 of 22 | |
|---|---|
| Drug Name | Isovue-300 |
| Drug Label | ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a sui... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Bracco |
| 18 of 22 | |
|---|---|
| Drug Name | Isovue-370 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Bracco |
| 19 of 22 | |
|---|---|
| Drug Name | Isovue-m 200 |
| Drug Label | ISOVUE (lopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration.Each mL of ISOVUE-200 (lopamidol Injection 41%) provides 408 mg iopamidol with 1 mg tromethamine and 0.26 mg edetate c... |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 41% |
| Market Status | Prescription |
| Company | Bracco |
| 20 of 22 | |
|---|---|
| Drug Name | Isovue-m 300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Bracco |
| 21 of 22 | |
|---|---|
| Drug Name | Scanlux-300 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 61% |
| Market Status | Prescription |
| Company | Sanochemia Corp Usa |
| 22 of 22 | |
|---|---|
| Drug Name | Scanlux-370 |
| Active Ingredient | Iopamidol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 76% |
| Market Status | Prescription |
| Company | Sanochemia Corp Usa |
/EXPERIMENTAL THER:/ Meconium obstruction of prematurity (MO) often occurs in extremely low-birth weight (ELBW) infants, and its treatment is quite a challenge for neonatologists. /Investigators/ attempted to establish a method of primary treatment for MO of prematurity in ELBW infants. An iopamidol enema with 50 cm H2O static pressure was performed as the primary treatment. This procedure is safe and effective and /was recommended/ as the first treatment for MO in ELBW infants. The procedure was performed 50 times in 23 infants and no complications occurred. Out of 23 patients, 20 (88%) improved, but the other 3 did not. In the failure group, the procedure was performed on a significantly later date and the mortality rate was higher (12.5 vs. 67%). This procedure is safe and effective. /The authors/ recommend this as the first treatment for MO in ELBW infants.
PMID:19184049 Nakaoka T et al; Pediatr Surg Int 25 (3): 273-6 (2009)
/EXPERIMENTAL THER:/ ... Ninety mice used in this study were divided into three groups: lipiodol, iopamidol, and normal saline. The test compounds were given by submucosal injection to the gastric wall of anesthetized mice. The specimens were subjected to histopathological examination. The mean grades of acute inflammatory response after iopamidol and lipiodol injection were significantly higher than control group. However, there was no significant difference between iopamidol and lipiodol injection. The mean grade of chronic inflammatory response and fibrosis showed no differences between groups. The presence or absence of fibrinoid necrosis and mesothelial hyperplasia showed no statistical differences at each time point between groups. The foam cell, which is similar to human signet ring cell carcinoma, were not identified in normal saline and iopamidol group, but were detected by postoperative day 7 in lipiodol group. /It was concluded/ that iopamidol and lipiodol when used as a contrast media of CT lymphography is an available material for preoperative sentinel node navigation surgery for gastric cancer with an acceptable incidence of pathological alterations in a mouse model. /The/ results are potentially useful to clinical (human) application.
PMID:22347708 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3278638 Hwang SH et al; J Korean Surg Soc 82 (2): 70-8 (2012)
Hematomas and intraparenchymal bleeders seldom demonstrate any contrast enhancement. However, in cases of intraparenchymal clot, for which there is no obvious clinical explanation, contrast media administration may be helpful in ruling out the possibility of associated arteriovenous malformation. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
Arteriovenous malformations and aneurysms will show contrast enhancement. For these vascular lesions, the enhancement is probably dependent on the iodine content of the circulating blood pool. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
For more Therapeutic Uses (Complete) data for Iopamidol (9 total), please visit the HSDB record page.
Focal and generalized motor seizures have been reported after intrathecal use of water-soluble contrast agents including iopamidol. In several of those cases reported with iopamidol, higher than recommended doses were employed. Therefore avoid: deviations from recommended neuroradiologic procedure or patient management; use in patients with a history of epilepsy unless medically justified; overdosage; intracranial entry of a bolus or premature diffusion of a high concentration of the medium; failure to maintain elevation of the head during the procedure, on the stretcher, and in bed; excessive and particularly active patient movement or straining.[US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE-M (iopamidol) injection, solution
Bracco Diagnostics Inc] (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=11e893d2-0183-4581-b908-c8b7302c7edb
FDA Pregnancy risk category B: NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absents of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility.
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
Use of medications that may lower the seizure threshold (phenothiazine derivatives, including those used for their antihistaminic properties; tricyclic antidepressants; MAO inhibitors; CNS stimulants; analeptics; antipsychotic agents) should be carefully evaluated. While the contributory role of such medications has not been established, some physicians have discontinued these agents at least 48 hours before and for at least 24 hours following intrathecal use.[US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE-M (iopamidol) injection, solution
Bracco Diagnostics Inc] (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=11e893d2-0183-4581-b908-c8b7302c7edb
Direct intracisternal or ventricular administration for standard radiography (without computerized tomographic enhancement) is not recommended. Inadvertent intracranial entry of a large or concentrated bolus of the contrast medium, which increases the risk of neurotoxicity, can be prevented by careful patient management. Also, effort should be directed to avoid rapid dispersion of the medium causing inadvertent rise to intracranial levels (e.g., by active patient movement). If such intracranial entry of the medium occurs, prophylactic anticonvulsant treatment with diazepam or barbiturates orally for 24 to 48 hours should be considered.[US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE-M (iopamidol) injection, solution
Bracco Diagnostics Inc] (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=11e893d2-0183-4581-b908-c8b7302c7edb
For more Drug Warnings (Complete) data for Iopamidol (37 total), please visit the HSDB record page.
FDA Label
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB04 - Iopamidol
Radiographic contrast media (CM) induce renal vasoconstriction and may initiate induced nephropathy. Endothelin (ET), a vasoconstrictor, and nitric oxide (NO), a vasodilator, which are synthesized in the kidney by the vascular endothelium as well as by tubular epithelial and glomerular mesangial cells, are key modulators of renal circulation after CM administration. Intravascular CM, in addition, induces pronounced diuresis and natriuresis. The aim of the present study was to evaluate and compare changes in endogenous vasoactive mediators and contrast-induced natriuresis after CM administration. Diagnostic angiographic procedures were performed in 14 patients (9 males and 5 females) using the non-ionic CM Iopamidol. Before and immediately after angiography, venous blood and urine samples were obtained. The urinary excretion of ET-1 and nitrates/nitrites (NOx), and the fractional excretion of sodium (FENa) were measured and analyzed. The urinary excretion of both ET-1 and NOx increased significantly (p < 0.05) after angiography, and urinary ET-1 and NOx excretion was correlated with an increase in FENa (p < 0.05). Exposure to CM in humans is associated with an increase in urinary ET and NOx. The excretion of sodium following CM administration is associated with an increase in urinary ET and NOx. ET and NO might be important in the renal change in humans after CM administration.
PMID:16015005 Murakami R et al; Nephron Clin Pract 101 (3): c150-4 (2005)
No iodinated compound other than Iopamidol was found in the urine of subjects who received intrathecal injection of 10 mL of Iopamiro "300". The compound was neither metabolized nor altered in its optical configuration and urinary iodide content was always in the normal range. Between 72 and 85% of injected Iopamidol was excreted within 72 h of injection.
PMID:6687926 Pitre D et al; Neuroradiology 25 (1): 37-8 (1983)
It is not known whether this drug is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
The pharmacokinetics of iopamidol in both normal and abnormal tissue have been shown to be variable. Contrast enhancement appears to be greatest soon after administration of the contrast medium, and following intraarterial rather than intravenous administration. ...
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
For more Absorption, Distribution and Excretion (Complete) data for Iopamidol (8 total), please visit the HSDB record page.
No significant metabolism, deiodination, or biotransformation occurs.
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
The pharmacokinetics of iopamidol 370 (Iopamiro), a non-ionic water soluble organic iodine compound, were studied in adults with different degrees of chronic renal failure and in healthy volunteers. After 50 mL were administered i.v., plasma and urine levels were determined. The main pharmacokinetic parameters were calculated on the basis of bi-compartmental open model. There were significant differences from healthy volunteers in half-life beta, which increased with the degree of renal failure as the clearance values decreased. Half-life beta was equal to 1.67 h in healthy volunteers, 4.24 h in patients with mild renal failure and 10.03 h in patients with severe renal failure. The clearance decreased as follows: 0.11 (L/h kg) in healthy volunteers, 0.06 (L/h kg) in patients with mild renal failure and 0.02 (L/h kg) in patients with severe renal failure. No significant differences were found in distribution volume values nor in half-life alpha.
PMID:2222559 Corradi A et al; Arzneimittelforschung 40 (7): 830-2 (1990)
The pharmacokinetics of intravenously administered iopamidol in normal subjects conform to an open two-compartment model with first order elimination (a rapid alpha phase for drug distribution and a slow beta phase for drug elimination). The elimination serum or plasma half-life is approximately two hours; the half-life is not dose dependent.
US Natl Inst Health; DailyMed. Current Medication Information for ISOVUE (iopamidol) injection, solution (January 2012). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=416ab996-a2e8-4fe1-a9c9-f10b86df3bb5
To test the hypothesis that iodinated contrast media may induce an elevation in serum potassium level, /contrast media was administered to rabbits and tested in-vitro according to four protocols./ Protocol A: After intravenous infusion of contrast media into six rabbits, alterations of potassium ion concentrations were measured. Protocol B: Fresh rabbit blood was mixed in vitro with contrast media, and the fluctuations in potassium were monitored over a 30-minute period. Protocol C: Similar to protocol B, except that blood from humans with no reaction to contrast media was used. For protocol A, blood potassium levels increased above baseline levels. The elevations were statistically significant (P < .05). For protocol B, diatrizoate and ioxaglate caused a gradual increase in blood potassium levels, but iopamidol did not. In protocol C, all three contrast media caused statistically significant elevation in potassium levels. The release of potassium was statistically significant at 5 minutes (P < .05 for diatrizoate and ioxaglate, and P < .01 for iopamidol). The mean release rates (+/- standard deviation) by means of linear regression analysis were 0.0190 mmol/min +/- 0.0112 with diatrizoate, 0.0159 mmol/min +/- 0.0057 with iopamidol, and 0.0088 mmol/min +/- 0.0033 with ioxaglate. Iodinated contrast media increase blood potassium levels causing release of potassium into intravascular spaces. This potassium release may play some role in contrast medium-induced adverse reactions.
PMID:8685334 Hayakawa K, Shimizu Y; Radiology 200 (2): 407-11 (1996)
The synthesis of prostaglandins and other metabolic products of arachidonic acid (AA) was investigated in isolated perfused lungs of hamsters during the infusion of various concentrations of meglumine diatrizoate and iopamidol. Forty nmol of (14)C-AA was infused into the pulmonary circulation with radiographic contrast media (RCM), and prostaglandins, thromboxanes, and metabolites of lipoxygenases were analyzed from the nonrecirculating perfusion effluent. Arachidonate infusion increased the perfusion pressure. This pressor response was decreased by iopamidol ... The amount of radioactivity was decreased in the perfusion effluent and increased in lung lipids by iopamidol. ... Almost all arachidonate metabolites were decreased significantly by iopamidol when compared with hypertonic saline ...
PMID:6413449 Paajanen H et al; Invest Radiol 18 (4): 375-81 (1983)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
78
PharmaCompass offers a list of Iopamidol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iopamidol manufacturer or Iopamidol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iopamidol manufacturer or Iopamidol supplier.
PharmaCompass also assists you with knowing the Iopamidol API Price utilized in the formulation of products. Iopamidol API Price is not always fixed or binding as the Iopamidol Price is obtained through a variety of data sources. The Iopamidol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Iopamidol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Iopamidol, including repackagers and relabelers. The FDA regulates Iopamidol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Iopamidol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Iopamidol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Iopamidol supplier is an individual or a company that provides Iopamidol active pharmaceutical ingredient (API) or Iopamidol finished formulations upon request. The Iopamidol suppliers may include Iopamidol API manufacturers, exporters, distributors and traders.
click here to find a list of Iopamidol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Iopamidol DMF (Drug Master File) is a document detailing the whole manufacturing process of Iopamidol active pharmaceutical ingredient (API) in detail. Different forms of Iopamidol DMFs exist exist since differing nations have different regulations, such as Iopamidol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Iopamidol DMF submitted to regulatory agencies in the US is known as a USDMF. Iopamidol USDMF includes data on Iopamidol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Iopamidol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Iopamidol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Iopamidol Drug Master File in Japan (Iopamidol JDMF) empowers Iopamidol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Iopamidol JDMF during the approval evaluation for pharmaceutical products. At the time of Iopamidol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Iopamidol suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Iopamidol Drug Master File in Korea (Iopamidol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Iopamidol. The MFDS reviews the Iopamidol KDMF as part of the drug registration process and uses the information provided in the Iopamidol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Iopamidol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Iopamidol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Iopamidol suppliers with KDMF on PharmaCompass.
A Iopamidol CEP of the European Pharmacopoeia monograph is often referred to as a Iopamidol Certificate of Suitability (COS). The purpose of a Iopamidol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Iopamidol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Iopamidol to their clients by showing that a Iopamidol CEP has been issued for it. The manufacturer submits a Iopamidol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Iopamidol CEP holder for the record. Additionally, the data presented in the Iopamidol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Iopamidol DMF.
A Iopamidol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Iopamidol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Iopamidol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Iopamidol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Iopamidol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Iopamidol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Iopamidol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Iopamidol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Iopamidol suppliers with NDC on PharmaCompass.
Iopamidol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Iopamidol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Iopamidol GMP manufacturer or Iopamidol GMP API supplier for your needs.
A Iopamidol CoA (Certificate of Analysis) is a formal document that attests to Iopamidol's compliance with Iopamidol specifications and serves as a tool for batch-level quality control.
Iopamidol CoA mostly includes findings from lab analyses of a specific batch. For each Iopamidol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Iopamidol may be tested according to a variety of international standards, such as European Pharmacopoeia (Iopamidol EP), Iopamidol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Iopamidol USP).

