

X


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
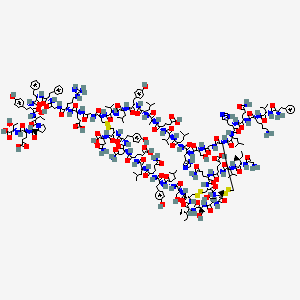

| Molecular Weight | 5823 g/mol |
|---|---|
| Molecular Formula | C258H384N64O78S6 |
| XLogP3 | -12.1 |
| Hydrogen Bond Donor Count | 78 |
| Hydrogen Bond Acceptor Count | 90 |
| Rotatable Bond Count | 180 |
| Exact Mass | 5820.6440255 g/mol |
| Monoisotopic Mass | 5818.6373159 g/mol |
| Topological Polar Surface Area | 2450 Ų |
| Heavy Atom Count | 406 |
| Formal Charge | 0 |
| Complexity | 14600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 52 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Apidra |
| Drug Label | APIDRA (insulin glulisine [rDNA origin] injection) is a rapid-acting human insulin analog used to lower blood glucose. Insulin glulisine is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12... |
| Active Ingredient | Insulin glulisine recombinant |
| Dosage Form | Injectable |
| Route | Iv (infusion), subcutaneous |
| Strength | 300 units/3ml (100 units/ml); 1000 units/10ml (100 units/ml) |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 4 | |
|---|---|
| Drug Name | Apidra solostar |
| PubMed Health | Insulin Glulisine (Injection) |
| Drug Classes | Antidiabetic |
| Drug Label | APIDRA (insulin glulisine [rDNA origin] injection) is a rapid-acting human insulin analog used to lower blood glucose. Insulin glulisine is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12... |
| Active Ingredient | Insulin glulisine recombinant |
| Dosage Form | Injectable |
| Route | Subcutaneous |
| Strength | 300 units/3ml |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 3 of 4 | |
|---|---|
| Drug Name | Apidra |
| Drug Label | APIDRA (insulin glulisine [rDNA origin] injection) is a rapid-acting human insulin analog used to lower blood glucose. Insulin glulisine is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12... |
| Active Ingredient | Insulin glulisine recombinant |
| Dosage Form | Injectable |
| Route | Iv (infusion), subcutaneous |
| Strength | 300 units/3ml (100 units/ml); 1000 units/10ml (100 units/ml) |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 4 of 4 | |
|---|---|
| Drug Name | Apidra solostar |
| PubMed Health | Insulin Glulisine (Injection) |
| Drug Classes | Antidiabetic |
| Drug Label | APIDRA (insulin glulisine [rDNA origin] injection) is a rapid-acting human insulin analog used to lower blood glucose. Insulin glulisine is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12... |
| Active Ingredient | Insulin glulisine recombinant |
| Dosage Form | Injectable |
| Route | Subcutaneous |
| Strength | 300 units/3ml |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |


