



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
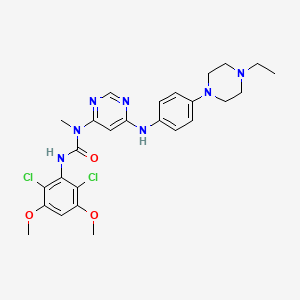

1. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-(4-(4-ethylpiperazin-1-yl)-phenylamino)pyrimidin-4-yl)-1-methylurea
2. Bgj398
3. Nvp-bgj398
4. Truseltiq
1. Nvp-bgj398
2. 872511-34-7
3. Bgj398
4. Bgj-398
5. Bgj 398
6. Truseltiq
7. Infigratinib [inn]
8. Infigratinib Free Base
9. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-((4-(4-ethylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-1-methylurea
10. Infigratinib [usan]
11. Bgj398 (nvp-bgj398)
12. Mvp-bgj398
13. A4055me1vk
14. Chebi:63451
15. 872511-34-7 (free Base)
16. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-{[4-(4-ethylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl)-1-methylurea
17. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-[6-[4-(4-ethylpiperazin-1-yl)anilino]pyrimidin-4-yl]-1-methylurea
18. Urea, N'-(2,6-dichloro-3,5-dimethoxyphenyl)-n-(6-((4-(4-ethyl-1-piperazinyl)phenyl)amino)-4-pyrimidinyl)-n-methyl-
19. Chembl1834657
20. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-((4-(4-ethylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-1-methylurea.
21. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-[6-[[4-(4-ethylpiperazin-1-yl)phenyl]amino]pyrimidin-4-yl]-1-methylurea
22. Unii-a4055me1vk
23. Infigratinib (bgj398)
24. Infigratinib (usan/inn)
25. Infigratinib [usan:inn]
26. Nvp-bgj389
27. Nvp-bgj398(infigratinib)
28. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-(4-(4-ethylpiperazin-1-yl)phenylamino)pyrimidin-4-yl)-1-methylurea
29. Mls006010953
30. Infigratinib [who-dd]
31. Schembl374435
32. Gtpl7877
33. Chembl1852688
34. Dtxsid70236238
35. Ex-a057
36. Bgj398, Bgj-398
37. Hms3295o21
38. Amy10737
39. Bcp03602
40. Bgj398 - Nvp-bgj398
41. Bdbm50355393
42. Fd5035
43. Mfcd22123241
44. Nsc764487
45. S2183
46. Who 10032
47. Zinc72105034
48. Akos025149513
49. Akos032949944
50. Bcp9000399
51. Cs-0586
52. Db11886
53. Nsc-764487
54. Sb16612
55. Ncgc00274030-01
56. Ncgc00274030-11
57. 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-(4-(4-ethylpiperazin-1-yl)-phenylamino)pyrimidin-4-yl)-1-methylurea
58. Ac-28417
59. As-16290
60. Hy-13311
61. Smr004702757
62. Bcp0726000187
63. Ft-0699366
64. D11589
65. J-510477
66. Brd-k42728290-001-01-8
67. Q27075200
68. 07j
69. 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea
70. N'-(2,6-dichloro-3,5-dimethoxyphenyl)-n-(6-((4-(4-ethyl-1- Piperazinyl)phenyl)amino)-4-pyrimidinyl)-n-methylurea
| Molecular Weight | 560.5 g/mol |
|---|---|
| Molecular Formula | C26H31Cl2N7O3 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 559.1865433 g/mol |
| Monoisotopic Mass | 559.1865433 g/mol |
| Topological Polar Surface Area | 95.1 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 724 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Infigratinib is indicated for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma in adults with a fibroblast growth factor receptor 2 (FGFR2) fusion or another rearrangement as detected by an FDA-approved test.
Treatment of cholangiocarcinoma
Treatment of achondroplasia
Infigratinib is an anti-tumour agent that works to suppress tumour growth in cholangiocarcinoma. It exhibits anti-tumour activity in mouse and rat xenograft models of human tumours with activating FGFR2 or FGFR3 alterations, such as FGFR2-TTC28 or FGFR2-TRA2B fusions. In clinical trials, patients with cholangiocarcinoma who were treated with infigratinib had an overall response rate of 23% - where one patient had a complete response - and a duration of response of 5.5 months, with a range between 0.03 and 28.3 months. Some patients with cancers with FGFR mutations display intrinsic resistance to infigratinib, leading to negligible therapeutic efficacy: investigations are ongoing to target molecular pathways to combat drug resistance.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EN - Fibroblast growth factor receptor (fgfr) tyrosine kinase inhibitors
L01EN03 - Infigratinib
Absorption
Mean (%CV) Cmax is 282.5 ng/mL (54%) and AUC0-24h is 3780 ngxh/mL (59%) for infigratinib. Infigratinib Cmax and AUC increase more than proportionally across the dose range of 5 to 150 mg and steady state is achieved within 15 days. At steady state, median time to achieve peak infigratinib plasma concentration (Tmax) is six hours, with a range between two and seven hours. Mean (%CV) Cmax is 42.1 ng/mL (65%) for BHS697 and 15.7 ng/mL (92%) for CQM157. Mean (%CV) AUC0-24h is 717 ngxh/mL (55%) for BHS697 and 428 ngxh/mL (72%) for CQM157. In healthy subjects, a high-fat and high-calorie meal increased AUCinf of infigratinib by 80%-120% and Cmax by 60%-80%. The median Tmax also shifted from four hours to six hours. A low-fat low-calorie meal increased the mean AUCinf of infigratinib by 70% and Cmax by 90%/
Route of Elimination
Following administration of a single oral dose of radiolabeled infigratinib in healthy subjects, approximately 77% of the dose was recovered in feces, where 3.4% of the dose was in the unchanged parent form. About 7.2% was recovered in urine with 1.9% of the dose was unchanged.
Volume of Distribution
At steady state, the geometric mean (CV%) apparent volume of distribution of infigratinib was 1600 L (33%). In rats receiving a single oral dose, infigratinib had brain-to-plasma concentration ratios (based on AUC0-inf) of 0.682.
Clearance
The geometric mean (CV%) total apparent clearance (CL/F) of infigratinib was 33.1 L/h (59%) at steady state.
According to _in vitro_ findings, about 94% of infigratinib is metabolized by CYP3A4 and about 6% of the drug is metabolized by flavin-containing monooxygenase 3 (FMO3). About 38% of the dose is circulating parent drug in the plasma and BHS697 and CQM157 are two major metabolites of infigratinib that are each found at >10% of the dose. They are pharmacologically active, with BHS697 representing about 16% to 33% of the overall pharmacological activity of infigratinib and CQM157 contributing to about 9% to 12%. BHS697 undergoes further metabolism mediated by CYP3A4 and CQM157 is metabolized through both Phase I and Phase II biotransformation pathways. The exact metabolic pathways and the structure of BHS697 and CQM157 are not fully characterized.
The geometric mean (CV%) terminal half-life of infigratinib was 33.5 h (39%) at steady state.
Fibroblast growth factor receptors (FGFRs) are tyrosine kinase receptors that play a role in cell proliferation, differentiation, migration, survival, and angiogenesis. Upon binding of extracellular signals, primarily fibroblast growth factors, FGFR dimerizes to promote phosphorylation of downstream molecules and activation of the Ras-mitogen-activated protein kinase (MAPK) pathway. In some cancers, the FGFR signalling pathway is aberrant and disrupted, leading to unregulated cell proliferation and growth, including malignant cells. Alterations in the FGFR receptors, including mutations, amplifications, and fusions, are associated with a wide array of neoplasms, including prostate, urothelial, ovarian, breast, and liver cancer. In particular, FGFR2 fusion is closely related to intrahepatic cholangiocarcinoma: recent studies show that up to 45% of patients with intrahepatic cholangiocarcinoma exhibited gene rearrangements resulting in FGFR2 fusion proteins. Alterations in FGFR in tumours can lead to constitutive FGFR signalling, supporting the proliferation and survival of malignant cells. Infigratinib is a reversible, non-competitive inhibitor of all four FGFR subtypes - FGFR1, FGFR2, FGFR3, and FGFR4 - that blocks FGFR signalling and inhibits cell proliferation in cancer cell lines with activating FGFR amplification, mutations, or fusions. Out of the four FGFR subtypes, infigratinib has the highest affinity for FGFR1, FGFR2, and FGFR3. Infigratinib binds to the allosteric site between the two kinase lobes of the FGFR - or more specifically, to the ATP-binding cleft. Binding to this cleft prevents autophosphorylation of the receptor and blocks downstream signalling cascades that would otherwise activate MAPK.


