
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Canada
0
Australia
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
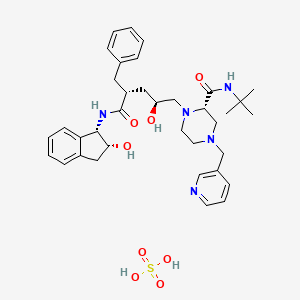

1. Crixivan
2. Indinavir
3. Indinavir, Sulfate (1:1)
4. L 735 524
5. L 735,524
6. L-735 524
7. L-735,524
8. L735 524
9. L735,524
10. Mk 639
11. Mk-639
12. Mk639
13. Sulfate, Indinavir
1. 157810-81-6
2. Crixivan
3. Indinavir Sulphate
4. Indinavir (sulfate)
5. Mk-639
6. Mk 639
7. L-735,524
8. Nsc-697197
9. L 735524
10. Chebi:5899
11. (s)-1-((2s,4r)-4-benzyl-2-hydroxy-5-(((1s,2r)-2-hydroxy-2,3-dihydro-1h-inden-1-yl)amino)-5-oxopentyl)-n-(tert-butyl)-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide Sulfate
12. Crixivan (tn)
13. 771h53976q
14. L-735524
15. Dsstox_cid_24221
16. Dsstox_rid_80126
17. Dsstox_gsid_44221
18. D-erythro-pentonamide, 2,3,5-trideoxy-n-(2,3-dihydro-2-hydroxy-1h-inden-1-yl)-5-(2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-, (1(1s,2r,5(s))-, Sulfate (1:1) (salt)
19. D-erythro-pentonamide, 2,3,5-trideoxy-n-[(1s,2r)-2,3-dihydro-2-hydroxy-1h-inden-1-yl]-5-[(2s)-2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-, Sulfate (1:1)
20. Indinavir Sulfate [usan]
21. (2s)-1-[(2s,4r)-4-benzyl-2-hydroxy-5-[[(1s,2r)-2-hydroxy-2,3-dihydro-1h-inden-1-yl]amino]-5-oxopentyl]-n-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide;sulfuric Acid
22. Smr000469161
23. Cas-157810-81-6
24. Nsc697197
25. Hsdb 7158
26. Drg-0233
27. Indinavir Sulfate [usan:usp]
28. Indinaviri Sulfas
29. Indinivar Sulphate
30. Unii-771h53976q
31. Ncgc00159460-02
32. Mfcd00920346
33. Indinavir Sulfate (usp)
34. Chembl1735
35. Schembl41040
36. (alphar,gammas,2s)-alpha-benzyl-2-(tert-butylcarbamoyl)-gamma-hydroxy-n-((1s,2r)-2-hydroxy-1-indanyl)-4-(3-pyridylmethyl)-1-piperazinevaleramide Sulfate (1:1) (salt)
37. Mls001401425
38. Mls006010223
39. Indinavir Sulfate [mi]
40. Indinavir For System Suitability
41. Dtxsid1044221
42. Indinavir Sulfate [hsdb]
43. Hy-b0689a
44. Indinavir Sulfate [vandf]
45. Indinavir Sulfate [mart.]
46. Hms2051p19
47. Hms2231g18
48. Hms3715l06
49. Indinavir Sulfate [who-dd]
50. Indinavir Sulfate [who-ip]
51. Tox21_111687
52. Tox21_302415
53. S9567
54. Indinavir Sulphate [ema Epar]
55. Akos015920149
56. Tox21_111687_1
57. Ac-1935
58. Ccg-100982
59. Indinavir Sulfate [orange Book]
60. Nc00232
61. Nsc 697197
62. Indinavir Sulfate [usp Monograph]
63. Indinaviri Sulfas [who-ip Latin]
64. Ncgc00159460-04
65. Ncgc00255764-01
66. As-30738
67. D-erythro-pentonamide, 2,3,5-trideoxy-n-((1s,2r)-2,3-dihydro-2-hydroxy-1h-inden-1-yl)-5-((2s)-2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-, Sulfate (1:1) (salt)
68. D-erythro-pentonamide, 2,3,5-trideoxy-n-(2,3-dihydro-2-hydroxy-1h-inden-1-yl)-5-(2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-, Monohydrate, (1(1s,2r),5(s))-, Sulfate (1:1)
69. C08089
70. D00897
71. 810i816
72. Q27106928
73. (.alpha.r,.gamma.s,2s)-.alpha.-benzyl-2-(tert-butylcarbamoyl)-.gamma.-hydroxy-n-((1s,2r)-2-hydroxy-1-indanyl)-4-(3-pyridylmethyl)-1-piperazinevaleramide Sulfate (1:1) (salt)
74. (.alpha.r,.gamma.s,2s)-.alpha.-benzyl-2-(tert-butylcarbamoyl)-.gamma.-hydroxy-n-((1s,2r)-2-hydroxy-1-indanyl)-4-(3-pyridylmethyl)-1-piperazinevaleramide Sulphate (1:1) (salt)
75. 2,3,5-trideoxy-n-[(1s,2r)-2,3-dihydro-2-hydroxy-1h-inden-1-yl]-5-[(2s)-2[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-d-erythro-pentonamide
76. D-erythro-pentonamide, 2,3,5-trideoxy-n-(2,3-dihydro-2-hydroxy-1h-inden-1-yl)-5-(2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-, (1(1s,2r,5(s))-, Sulphate (1:1) (salt)
77. D-erythro-pentonamide,3,5-trideoxy-n-(2,3-dihydro-2- Hydroxy-1h-inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino] Carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-, [1(1s,2r),5(s)-, Sulfate (1:1) (salt)
| Molecular Weight | 711.9 g/mol |
|---|---|
| Molecular Formula | C36H49N5O8S |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Exact Mass | 711.33018471 g/mol |
| Monoisotopic Mass | 711.33018471 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 1030 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Crixivan |
| PubMed Health | Indinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | CRIXIVANRegistered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.Copyright 1996, 1997, 1998, 1999, 2004 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved (indinavir sulfate) is an i |
| Active Ingredient | Indinavir sulfate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 2 of 2 | |
|---|---|
| Drug Name | Crixivan |
| PubMed Health | Indinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | CRIXIVANRegistered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.Copyright 1996, 1997, 1998, 1999, 2004 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved (indinavir sulfate) is an i |
| Active Ingredient | Indinavir sulfate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
HIV Protease Inhibitors.
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Indinavir with antiretroviral agents is indicated for the treatment of HIV infection. /Included in US product labeling/
Drug Facts and Comparisons 2011. Wolters Kluwer Health St. Louis, MO 2011, p. 2449
Nephrolithiasis/urolithiasis, which may present as flank pain with or without hematuria (including microscopic hematuria), has been reported in about 9% of adults and 29% of pediatric patients receiving indinavir.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
The most frequent adverse effects associated with indinavir therapy involve the GI tract. ...In treatment-naive HIV-infected adults, abdominal pain, nausea, vomiting, and diarrhea occurred in 16.6, 11.7, 8.4, and 3.3%, respectively, and acid regurgitation, anorexia, dyspepsia, increased appetite, and taste perversion occurred in 1.5-2.7% of patients receiving indinavir monotherapy. In patients in study 028 receiving indinavir in conjunction with zidovudine, abdominal pain, nausea, vomiting, and diarrhea occurred in 16, 31.9, 17.8, and 3%, respectively, and acid regurgitation, anorexia, dyspepsia, increased appetite, and taste perversion occurred in 1.5-8.4% of patients. ...Safety and efficacy of indinavir in pediatric patients have not been established. Indinavir has been used in a limited number of HIV-infected children 3 months of age or older without unusual adverse effects. However, nephrolithiasis/urolithiasis has been reported more frequently in pediatric patients receiving indinavir (29%) than in adults receiving the drug (9.2%).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Asymptomatic hyperbilirubinemia (i.e., total serum bilirubin concentrations exceeding 2.5 mg/dL) has occurred in about 14% of patients receiving indinavir in clinical studies. Asymptomatic bilirubinemia usually has been reported as elevated indirect bilirubin and has been associated with increased serum AST (SGOT) or ALT (SGPT) concentrations only rarely (i.e., in less than 1% of patients receiving the drug).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Acute hepatitis sometimes resulting in hepatic failure and death have been reported in a few patients receiving indinavir in conjunction with other drugs. ...Jaundice was reported in 1.5-2.1% of patients receiving indinavir.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
For more Drug Warnings (Complete) data for INDINAVIR SULFATE (21 total), please visit the HSDB record page.
Crixivan is indicated in combination with antiretroviral nucleoside analogues for the treatment of HIV-1 infected adults.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05AE02
In a study in HIV-infected children 4-17 years of age receiving an antiretroviral regimen that included oral indinavir (initial dosage of 500 mg/sq m every 8 hours; subsequent dosage averaging 2043 mg/sq m daily in 3 or 4 doses); peak and trough plasma concentrations averaging 7.3 and 0.29 ug/ml, respectively.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Indinavir is rapidly absorbed after oral administration, with peak levels achieved in approximately 1 hour. Unlike other drugs in this class, food can adversely affect indinavir bioavailability; a high-calorie, high-fat meal reduces plasma concentrations by 75%.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1654
Indinavir is excreted principally in the feces, both as unabsorbed drug and metabolites. Following oral administration of 400 mg of radiolabeled indinavir, 83% of the dose is recovered in feces (19.1% as unchanged drug) and 19% is recovered in urine (9.4% as unchanged drug). Following oral administration of a single 700- or 1000-mg dose of indinavir, 10.4 or 12%, respectively, is excreted unchanged in urine.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
To characterize steady-state indinavir pharmacokinetics in cerebrospinal fluid and plasma, 8 adults infected with human immunodeficiency virus underwent intensive cerebrospinal fluid sampling while receiving indinavir (800 mg every 8 hours) plus nucleoside reverse transcriptase inhibitors. Nine and 11 serial cerebrospinal fluid and plasma samples, respectively, were obtained from each subject. Free indinavir accounted for 94.3% of the drug in cerebrospinal fluid and 41.7% in plasma. Mean values of cerebrospinal fluid peak concentration, concentration at 8 hours, and area under the concentration-time profile calculated over the interval 0 to 8 hours (AUC(0-8)) for free indinavir were 294 nmol/L, 122 nmol/L, and 1616 nmol/L x hr, respectively. The cerebrospinal fluid-to-plasma AUC(0-8) ratio for free indinavir was 14.7% +/- 2.6% and did not correlate with indexes of blood-brain barrier integrity or intrathecal immune activation. Indinavir achieves levels in cerebrospinal fluid that should contribute to control of human immunodeficiency virus type 1 replication in this compartment. The cerebrospinal fluid-to-plasma AUC (0-8) ratio suggests clearance mechanisms in addition to passive diffusion across the blood-cerebrospinal fluid barrier, perhaps by P-glycoprotein-mediated efflux.
PMID:11061576 Haas DW et al; Clin Pharmacol Ther 68 (4): 367-74 (2000)
For more Absorption, Distribution and Excretion (Complete) data for INDINAVIR SULFATE (7 total), please visit the HSDB record page.
Indinavir is metabolized to at least 7 metabolites including 1 glucuronide conjugate and 6 oxidative metabolites. Major metabolic pathways identified include glucuronidation at the pyridine nitrogen, pyridine N-oxidation, para-hydroxylation of the phenylmethyl group, 3-hydroxylation of the indan, and N-depyridomethylation. In vitro studies indicate that cytochrome P-450 isoenzyme CYP3A4 is the major enzyme involved in the formation of the oxidative metabolites.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
In a study in adults with cirrhosis and mild to moderate hepatic impairment, the elimination half-life of the drug was prolonged to 2.8 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
The plasma half-life of indinavir averages 1.8 hours. In HIV-infected children 4-17 years of age receiving an antiretroviral regimen that included oral indinavir, plasma half-life of the drug averaged 1.1 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Combinations of antiretroviral drugs are successfully used for the treatment of acquired immune deficiency syndrome and reduce the incidence of severe human immunodeficiency virus (HIV)-associated dementia. To test whether such drugs affect the GSH metabolism of brain cells, we have exposed astrocyte-rich primary cultures to various antiretroviral compounds. Treatment of the cultures with the protease inhibitors indinavir or nelfinavir in low micromolar concentrations resulted in a time- and concentration-dependent depletion of cellular GSH from viable cells which was accompanied by a matching increase in the extracellular GSH content. In contrast, the reverse transcriptase inhibitors zidovudine, lamivudine, efavirenz or nevirapine did not alter cellular or extracellular GSH levels. Removal of indinavir from the medium by washing the cells terminated the stimulated GSH export immediately, while the nelfinavir-induced accelerated GSH export was maintained even after removal of nelfinavir. The stimulation of the GSH export from viable astrocytes by indinavir or nelfinavir was completely prevented by the application of MK571, an inhibitor of the multidrug resistance protein 1. These data demonstrate that indinavir and nelfinavir stimulate multidrug resistance protein 1-mediated GSH export from viable astrocytes and suggest that treatment of patients with such inhibitors may affect the GSH homeostasis in brain.
PMID:22017299 Brandmann M et al; J Neurochem 120 (1): 78-92 (2012)
Indinavir is a selective, competitive, reversible inhibitor of HIV protease. HIV protease, an aspartic endopeptidase that functions as a homodimer, plays an essential role in the HIV replication cycle and the formation of infectious virus. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes (i.e., p55 and p160) to form structural proteins of the virion core (i.e., p17, p24, p9, and p7) and essential viral enzymes (i.e., reverse transcriptase, integrase, and protease). Because indinavir is a structural analog of the HIV Phe-Pro protease cleavage site, the drug inhibits the function of the enzyme. By interfering with the formation of these essential proteins and enzymes, indinavir blocks maturation of the virus and causes the formation of nonfunctional, immature, noninfectious virions. Indinavir is active in both acutely and chronically infected cells since it targets the HIV replication cycle after translation and before assembly. Thus, the drug is active in chronically infected cells (e.g., monocytes and macrophages) that generally are not affected by nucleoside reverse transcriptase inhibitors (e.g., didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Indinavir does not affect early stages of the HIV replication cycle; however, the drug interferes with the production of infectious HIV and limits further infectious spread of the virus.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
While the complete mechanisms of antiviral activity of indinavir have not been fully elucidated, indinavir apparently inhibits replication of retroviruses, including human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2), by interfering with HIV protease. The drug, therefore, exerts a virustatic effect against retroviruses by acting as an HIV protease inhibitor.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Indinavir is a highly specific inhibitor of HIV protease and does not appear to interfere with the activity of human aspartic endopeptidases at clinically relevant concentrations. In one study, there was no evidence of inhibition of human cathepsin D or renin using indinavir concentrations exceeding 10 uM.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Unlike nucleoside antiretroviral agents, the antiviral activity of indinavir does not depend on intracellular conversion to an active metabolite. Indinavir and other HIV protease inhibitors (e.g., amprenavir, lopinavir, nelfinavir, ritonavir, saquinavir) act at a different stage of the HIV replication cycle than nucleoside and nonnucleoside reverse transcriptase inhibitors, and results of in vitro studies indicate that the antiretroviral effects of HIV protease inhibitors and some nucleoside agents (e.g., didanosine, zidovudine) or nonnucleoside agents (e.g., efavirenz, nevirapine) may be additive or synergistic. In one in vitro study, indinavir used in conjunction with zidovudine resulted in an additive effect against HIV-1; however, addition of lamivudine to these drugs resulted in a synergistic effect.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
19
PharmaCompass offers a list of Indinavir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Indinavir manufacturer or Indinavir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Indinavir manufacturer or Indinavir supplier.
PharmaCompass also assists you with knowing the Indinavir API Price utilized in the formulation of products. Indinavir API Price is not always fixed or binding as the Indinavir Price is obtained through a variety of data sources. The Indinavir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Indinavir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Indinavir, including repackagers and relabelers. The FDA regulates Indinavir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Indinavir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Indinavir supplier is an individual or a company that provides Indinavir active pharmaceutical ingredient (API) or Indinavir finished formulations upon request. The Indinavir suppliers may include Indinavir API manufacturers, exporters, distributors and traders.
click here to find a list of Indinavir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Indinavir DMF (Drug Master File) is a document detailing the whole manufacturing process of Indinavir active pharmaceutical ingredient (API) in detail. Different forms of Indinavir DMFs exist exist since differing nations have different regulations, such as Indinavir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Indinavir DMF submitted to regulatory agencies in the US is known as a USDMF. Indinavir USDMF includes data on Indinavir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Indinavir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Indinavir suppliers with USDMF on PharmaCompass.
Indinavir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Indinavir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Indinavir GMP manufacturer or Indinavir GMP API supplier for your needs.
A Indinavir CoA (Certificate of Analysis) is a formal document that attests to Indinavir's compliance with Indinavir specifications and serves as a tool for batch-level quality control.
Indinavir CoA mostly includes findings from lab analyses of a specific batch. For each Indinavir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Indinavir may be tested according to a variety of international standards, such as European Pharmacopoeia (Indinavir EP), Indinavir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Indinavir USP).

